Meis1 regulates proliferation of non-small-cell lung cancer cells
Introduction
Meis1 is a member of TALE (3-amino-acid loop extension) family of homeodomain transcription factors, which interacts with Hox transcription factors and promotes their target genes expression (1). Previous study has indicated that Meis1 activates HIF1α and HIF2α to inhibit ROS generation in hematopoietic stem cells (2,3). Meis1 deficiency resulted in underdeveloped hematopoietic stem cells compartment and embryonic vascular patterning (4-6). The roles of Meis1 in cancer were revealed by research on myeloid leukemia cells (7,8). These studies have proved a proliferative role of Meis1 in myeloid leukemia cells (9,10). Furthermore, Meis1 was also accumulated in neuroblastoma (11-13). Therefore, a putative oncogenic role of Meis1 has been postulated (14). However, Meis1 also inhibits neonatal and adult cardiomyocytes proliferation by modulating cell cycle progression (15). These data suggest complicated roles of Meis1 in cell proliferation.
Lung and bronchus is one of the three most common cancers in both men and women. It has been reported that more than 20% death was caused by lung cancer in all Americans which are expected to die of cancer (16). Briefly, lung cancer is subgrouped into small cell lung cancer and non-small-cell lung cancer (NSCLC). Although chemotherapy, radiotherapy and surgical resection have some benefits for NSCLC, these traditional strategies remain inefficient. To shed new lights on NSCLC treatment, mechanistic insight into NSCLC development and progression are urgently demanded, which also implies novel molecular and cellular targets for treatment.
In the current search, we demonstrated an inhibitory role of Meis1 in NSCLC cell proliferation. Down-regulation of Meis1 resulted in induced proliferation in NSCLC cells. Cell cycle analysis indicated enhanced cell cycle progression after Meis1 expression was compromised. Accordingly, ectopic expression of Meis1 plasmid repressed the proliferation of NSCLC cells. Taken together, NSCLC cell proliferation is limited by Meis1.
Methods
Plasmids, cell culture and stable cell line construction
NSCLC adenocarcinoma cancer cell line A549 was purchased from ATCC and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone) containing 10% FBS (Hyclone). SPC-A1 was purchased from cell bank of Chinese Academic of Sciences. Full length of Meis1 cDNA was obtained and then was cloned into pLNCX plasmid. After transfected into 293 packing cells, the supernatant was added directly into A549 cells. After 48 h, infected cells were selected with neomycin.
RNA interference (RNAi)
Meis1 RNAi (gcucaguagcuuaagggaaTT and uucccuuaagcuacugagcTT) was synthesized in GenePharma (Shanghai, China). Cells (5×105) were incubated in 35-mm plates with mixture of RNAi (final concentration, 50 nM) and Lipofectamine RNAiMAX Reagent (Invitrogen) in serum-free medium for 6 h. Cells were then grown in media with 10% FBS.
EdU
After treated as described, cells were incubated with 10 μM EdU for 8 h and then fixed by 4% paraformaldehyde. EdU was detected with Click-iT EdU cell proliferation kit following the instruction (Invitrogen). Nuclei were visualized by DAPI.
Cell proliferation assay
Approximate 8,000 cells treated were plated into each well of 96-well culture plates. The cell medium was replaced with 20 μL 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) (5 mg/mL) (Molecular Probes) in DMEM. After incubation at 37 °C for 2 h, the MTT solution was removed. A total of 100 μL DMSO was added to dissolve precipitate for 10 min at room temperature. Absorbance was recorded at 540 nm using a Spectramax M2 microplate reader (Molecular Devices).
qRT-PCR
RNA was isolated by TRIzol (Invitrogen). cDNA synthesis was performed with the High Capacity cDNA Archive Kit (Applied Biosystems) following the manufacturer’s instructions. Transcript levels were determined using the StepOne/StepOnePlus™ Real-time PCR System (Applied Biosystems) using SYBR Green PCR Master mix according to the manufacturer’s instructions. The primer sequences are Meis1: F-TCCCCAGCACAGGTGACGATGAT, R-CTTCCCCCTTGCTTTGCGATTGGT; Arf: F-AAATGAGAAGAAAGAAACC, R-GTTGTAATAGGAGTGGAA and Cdkn1a: F-AGGTGGACCTGGAGACTC, R-CGGCGTTTGGAGTGGTAG.
Western blotting
Cells were collected and washed with ice-cold PBS and lyzed in lysis buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.25% Triton-X 100, phosphatase inhibitors cocktail and protein inhibitor cocktail) for 30 min on ice and then centrifuged (13,000 g, 10 min, 4 °C). After centrifugation, the supernatants were collected. The blots were reacted with antibodies for Meis1 (Santa Cruz Biotechnology) followed by horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson ImmunoResearch Laboratories). Chemiluminescence was detected with ECL western blot detection kits (Cell Signaling Technology).
Immunofluorescence
Cells were fixed with 4% paraformaldehyde, permeabilized with PBST (PBS plus 0.1% Triton X-100) and blocked with 2% BSA plus 1% goat or donkey serum in PBT. Slides were immunostained with primary antibody in blocking solution as indicated. The species-specific fluorescent secondary IgG (Invitrogen) were also diluted in blocking solution. After counterstained with DAPI, the images were captured by florescence microscope (Leica).
Statistical analysis
A total of 3 to 5 replicates were performed for all of the experiments. The data are presented as the mean and standard error of the mean (SD). Comparisons within groups were conducted using a t-test with repeated measures; the P values indicated in the figures are <0.05 (*), <0.01 (**) and <0.001 (***).
Results
Specific down-regulation of mesi1 in NSCLC cancer cells
The roles of Meis1 in NSCLC adenocarcinoma remain poorly understood. In order to explore this, we utilized RNAi to repress the expression of endogenous Meis1 in A549 cells. An RNAi sequence was selected and then examined for its inhibitory efficiency. Compared to scrambled RNAi, Both mRNA and protein levels of Meis1 were largely decreased by RNAi (Figure 1).

Meis1 inhibits NSCLC cell proliferation
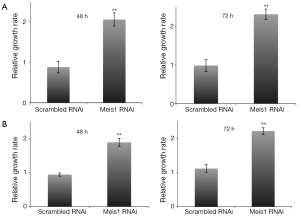
We further examined whether Meis1 affects NSCLC adenocarcinoma cell proliferation. We transfected Meis1 RNAi into two NSCLC cell lines A549 and SPC-A1. In A549 cells, down-regulation of Meis1 resulted in increased proliferation (Figure 2A). A comparable increase was also observed in SPC-A1 cells (Figure 2B). These data indicated that Meis1 represses NSCLC cancer cell proliferation.
Meis1 inhibit cell cycle entry of NSCLC cancer cell
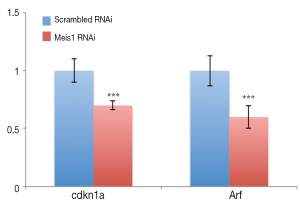
If compromised Meis1 expression results in increased proliferation, the cell cycle progression is supposed to be accelerated. To test this hypothesis, we then examined S phase entry by monitoring DNA synthesis. EdU is a nucleoside analog that can be incorporated into newly synthesized DNA to indicate DNA synthesis. We then monitored EdU incorporation. As expected, increased EdU incorporation was observed after Meis1 expression was compromised in both A549 and SPC-A1 (Figure 3). Consistently, increased cyclin D1 was also detected (Figure 3B). Furthermore, the mRNA levels of Cdkn1a and Arf, two CDK inhibitors were significantly down-regulated after silencing the expression of Meis 1 in A549 lung cancer cells (Figure 4). These data indicated Meis1 inhibits the cell cycle entry of NSCLC cancer cell. To further explore the whole cell cycle progression, we examined the mitosis by detecting the phosphorylation of Histone H3 at serine10, a marker of M phase. Consistent with the increased DNA synthesis, increased mitosis was observed after Meis1 expression was inhibited, denoted by Histone H3 at serine10 phosphorylation (Figure 3C). Collectively, these data indicated that Meis1 regulates cell cycle progression in NSCLC cancer cell.

NSCLC cancer cell proliferation was compromised by ectopic Meis1 expression
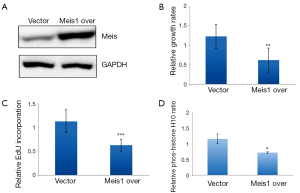
As endogenous Meis1 inhibits NSCLC cancer cell proliferation, we wondered to know whether increased Meis1 expression inhibits NSCLC cancer cell proliferation. We then established a Meis1 stably expressed A549 cells. Induced Meis1 expression was confirmed (Figure 5A). By using this cell line, we found that the proliferative rate was decreased compared to empty vector-transfected cells (Figure 5B). Similarly, DNA synthesis was down-regulated in Meis1 overexpressed A549 cells (Figure 5C). Consistently, reduced phosphorylation of Histone H3 at serine10 was observed in Meis1 overexpressed A549 cells (Figure 5D). Collectively, these data indicated increased Meis1 can inhibit NSCLC cancer cell proliferation.
Discussion
Meis1 has already been linked with proliferation in both normal and cancerous cells. In normal hematopoiesis stem cells and cardiomyocytes, Meis1 functions as an inhibitory protein to regulate these cell proliferations (15). The putative mechanisms for this regulation of Meis1 are modulation of cell cycle progression. Meis1 also limits cell cycle entry of hematopoietic stem cells, which in turn contributes to hematopoietic stem cells compartment (2). Furthermore, the expressions of cdkn1a and Ink4b-Arf-INK4a were largely compromised in Meis1 deficient cardiomyocytes, which then lead to cell cycle entry (15). In contrast, Meis1 exhibits a proliferative role in several cancerous cells such as myeloid leukemia cells and neuroblastoma (10,13). In myeloid leukemia cells, Meis1 cooperates with Hox9 and Pbx to activate the expression of Bmi1 and c-Myb, which then promote the proliferation (10). These observations suggest oncogenic roles of Meis1 in cancerous cells. However, our data indicated that Meis1 exhibits an inhibitory role for NSCLC cancer cell, suggesting the functions of Meis1 in cancerous are also diverse. These data indicate complicated roles of Meis1 in proliferation which may largely depend on cell context.
In the present study, we examined the regulation of cell cycle progression by Meis1 in NSCLC cancer cell. The EdU and cyclin D1 analysis indicated that cell cycle entry was indeed increased when Meis1 expression was compromised. This situation is similar with the alterations in hematopoiesis stem cells and cardiomyocytes induced by Meis1 deficiency, indicating a role of Meis1 in promoting cell quiescence to suppress the cell cycle entry. As a transcriptional co-regulator, Meis1 might affect cell cycle machinery by promoting downstream genes expression. As a transcriptional factor, the regulation of proliferation by Meis1 is possibly due to its transcriptional targets. Previous studies have indicated Meis1 promotes expression of CDK inhibitors. In neonatal cardiomyocytes, Meis1 promotes the up-regulation of Cdkn1a and Arf to limit the proliferative potential. Thus, it is reasonable to hypothesize that similar mechanisms may also exist in NSCLC cancer cells. In our experiment, we therefore measured the expression of those two genes in mRNA level in A549 cells. Our results demonstrated that significantly down-regulated Cdkn1a and Arf levels were observed after silencing the expression of Meis 1 in A549 lung cancer cells, suggesting the possible roles of CDK inhibitors in Meis 1 regulated proliferation in lung cancer. However, the underlying mechanisms for those CDK inhibitors in Meis 1-mediated cell cycle entry remain to be investigated.
Conclusions
Collectively, the data presented here indicated a repressive role of Meis1 in regulating NSCLC cancer cell proliferation through modulating cell cycle progression. It is intriguing to explore whether the expression levels of Meis1 was inversely correlated with the NSCLC cancer progression. If so, our data revealed a novel mechanism for the proliferation of NSCLC cancer cells and suggest a potential target for the development of future therapeutic strategies to treat NSCLC.
Acknowledgements
Disclosure: The authors declare no conflict of interests.
References
- Moens CB, Selleri L. Hox cofactors in vertebrate development. Dev Biol 2006;291:193-206. [PubMed]
- Kocabas F, Zheng J, Thet S, et al. Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood 2012;120:4963-72. [PubMed]
- Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010;7:380-90. [PubMed]
- Azcoitia V, Aracil M, Martínez-A C, et al. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol 2005;280:307-20. [PubMed]
- Hisa T, Spence SE, Rachel RA, et al. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO J 2004;23:450-9. [PubMed]
- Pillay LM, Forrester AM, Erickson T, et al. The Hox cofactors Meis1 and Pbx act upstream of gata1 to regulate primitive hematopoiesis. Dev Biol 2010;340:306-17. [PubMed]
- Thorsteinsdottir U, Kroon E, Jerome L, et al. Defining roles for HOX and MEIS1 genes in induction of acute myeloid leukemia. Mol Cell Biol 2001;21:224-34. [PubMed]
- Kawagoe H, Humphries RK, Blair A, et al. Expression of HOX genes, HOX cofactors, and MLL in phenotypically and functionally defined subpopulations of leukemic and normal human hematopoietic cells. Leukemia 1999;13:687-98. [PubMed]
- Lawrence HJ, Rozenfeld S, Cruz C, et al. Frequent co-expression of the HOXA9 and MEIS1 homeobox genes in human myeloid leukemias. Leukemia 1999;13:1993-9. [PubMed]
- Wong P, Iwasaki M, Somervaille TC, et al. Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes Dev 2007;21:2762-74. [PubMed]
- Jones TA, Flomen RH, Senger G, et al. The homeobox gene MEIS1 is amplified in IMR-32 and highly expressed in other neuroblastoma cell lines. Eur J Cancer 2000;36:2368-74. [PubMed]
- Geerts D, Schilderink N, Jorritsma G, et al. The role of the MEIS homeobox genes in neuroblastoma. Cancer Lett 2003;197:87-92. [PubMed]
- Spieker N, van Sluis P, Beitsma M, et al. The MEIS1 oncogene is highly expressed in neuroblastoma and amplified in cell line IMR32. Genomics 2001;71:214-21. [PubMed]
- Argiropoulos B, Yung E, Humphries RK. Unraveling the crucial roles of Meis1 in leukemogenesis and normal hematopoiesis. Genes Dev 2007;21:2845-9. [PubMed]
- Mahmoud AI, Kocabas F, Muralidhar SA, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 2013;497:249-53. [PubMed]
- Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin 1999;49:8-31, 1.


