
Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience
Introduction
During the past several years minimally invasive thoracic surgery has evolved from thoracoscopic approaches using 3–4 ports to a single incision video-assisted thoracoscopic surgery (VATS) techniques (1-3). Recently, the experience acquired with the uniportal VATS technique through the intercostal space has allowed the development of use of a uniportal VATS subxiphoid or subcostal approach for major pulmonary resections (4,5). The advantage of using a subxiphoid or subcostal entry is to reduce pain by avoiding possible trauma of intercostal nerves caused by thoracic incisions. However, the longer distance from the subxiphoid or subcostal incision to the hilum makes this approach more difficult to perform major pulmonary resections. Nevertheless, in expert hands this technique allows the possibility to perform complex resections (6), lymph node dissection (7) and anatomic segmentectomies (8).
During this same period of evolution into uniportal VATS surgery, robotic thoracic surgery has gained popularity as an alternative to traditional VATS. In some areas of the world such as the United States, robotic multiport thoracic surgery is more popular than uniportal VATS (9).The advantages of robotics are the ability to perform surgery more precisely with articulated or wristed instruments, motion scaling, and tremor filtration, as well as improved visualization thanks to 3D high definition video. However, currently 4–5 incisions are still necessary to perform anatomic robotic resections through the interspaces (10).
Recently, there has been a convergence of these two trends—uniportal surgery and robotic-assisted surgery—and has resulted in a single port robotic system, the da Vinci SP by Intuitive Surgical (Sunnyvale, California, USA). The single port (SP) platform is notable for a single 2.5 cm cannula through which an articulating 3D camera and 3 fully articulating instruments with 7 degrees of freedom can be passed. With its commercial introduction, we became interested in developing a robotic uniportal thoracic application by conducting experimental cadaver labs in the research setting. As of this writing (December 2018) the da Vinci SP has Food and Drug Administration (FDA) clearance in the US for urological procedures only, and the contents of this chapter are based on cadavers.
Overview of the da Vinci SP system
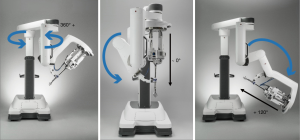
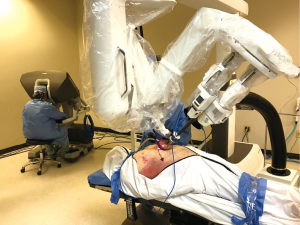
Like the conventional multiportal da Vinci Xi or X robotic platform, the SP system includes a free-standing surgeon console, a vision cart, and the patient side cart that consists of a single arm that controls up to 3 wristed instruments and the 3D articulating camera (Figure 1).
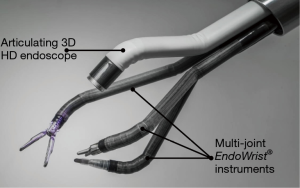
Movements performed by the surgeon’s hands, wrists, and arms in the console are mimicked by the robotic instruments and camera. Similar to da Vinci Xi and X technology, this allows range of motion to be beyond what is capable with the human hand inside the chest cavity, thanks to 7 degrees of freedom (rotation, in-out, pitch, yaw, grasp, wristed pitch, and wristed yaw) (Figure 2). The surgeon manipulates 2 master controls at the console and accordingly the finger and hand movements of the surgeon the sensors transmit the same movements inside the body. In addition, the system adds more precisión than VATS thanks to 3D magnified high definition video, the elimination of physiologic tremor, and the use of scaling from the motion of the surgeon movement to the surgical instruments which allows large motion to be scaled down to small motion.
Subxiphoid or subcostal incision
Based on our uniportal VATS surgical experience we sought to develop a uniportal robotic approach to perform thoracic procedures through a single incision to decrease pain. Due to the size of the trocar (2.5 cm) and the characteristic of the new platform (Figure 3) we concluded that this would not be an easy and reliable fit in the interspaces, and that the most suitable approach was to develop the procedure through the subxiphoid (preferred for thymectomy) or subcostal space (preferred for lobectomy).
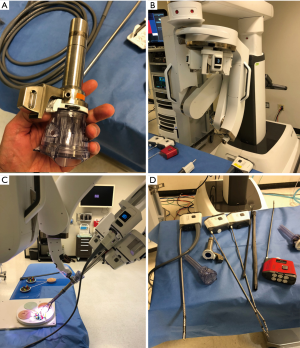
For major pulmonary resections, based on cadaver experiments, we believe the patient should be placed in a lateral decubitus position or semi-decubitus position (60–70 degrees inclination), similar to positioning for the subxiphoid VATS or intercostal approach (Figure 4). For thymectomy, the ideal approach is in the supine position. Moreover, in the supine position it may be advantageous to flex the bed to open more space in the epigastric region.
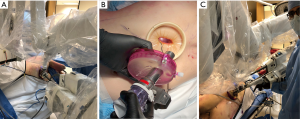
For the subxiphoid approach, a 4 cm vertical incision is made just over the prominence of the xiphoid process. The subcutaneous tissue is opened, and the rectus muscles are incised near the insertions to the costal arches at the midline. The cartilaginous xiphoid process is excised using scissors. The anterior mediastinum is opened from below the sternum, and a retrosternal tunnel is created by blunt finger dissection to open the pleural cavity. Importantly, the diaphragm is not violated in order to prevent the risk of a future diaphragmatic hernia. An access gel port is placed (GelPOINT, Applied Medical Corporation, Rancho Santa Margarita, California, USA) through which the 2.5 cm SP robotic trocar is placed (Figure 4A). The GelPoint access port allows CO2 insufflation, which increases the space and visibility in the chest and should be set to a pressure of 6–8 mmHg.
For the subcostal approach, an incision is placed lateral to the xiphoid process approximately 1 cm and is parallel to the subcostal margin (in the oblique direction). (Figure 4B). For pulmonary resection, an additional 12 mm trocar is inserted through the GelPOINT device side by side to the SP robotic trocar for the assistant to insert staplers, for suctioning, or to facilitate lung retraction and exposure if needed, especially for complex surgeries (Figure 4C).
Pros and cons
Based on our observations in cadavers, the da Vinci SP system overcomes many of the limitations of subxiphoid or subcostal VATS surgery. Compared to a subxiphoid thoracoscopic technique the robotic system provides several advantages, which we will describe below:
- The whole lung exposure and dissection (except stapler insertion) is performed by a single operator confortably seated in the console.
- Unlike with VATS where the instruments are coming in parallel with the camera, the articulation of the instrument elbows and wrists as well as articulation of the camera creates more natural triangulation to the area of interest.
- The enhanced view provided by the robotic platform is one the most important benefits. The 3D view with depth perception is a remarkable improvement over conventional VATS cameras. The ability of the surgeon to have full control the camera directly in a stable manner, with better maneuverability and magnification of the field is also an important advantage.
- The design of the robotic platform also filters unintended movements caused by physiologic tremor. This increases dexterity, restores proper hand-eye coordination and the surgeon maintains a natural ergonomic position.
- It eliminates the fulcrum effect of VATS instruments, making the instrument manipulation more ergonomic and intuitive.
- Based on these advances, we believe the SP system will allow the possibility to perform complex robotic procedures not easily possible with the conventional thoracoscopic subxiphoid approach, such as the sleeve bronchial and vascular resections.
- An extensive and complete lymph node dissection is very difficult to achieve via subxiphoid or subcostal VATS approach; however the design of the SP system with its snake-like configuration makes this step of procedure easier. We were able to expose the deeper subcarinal and paratracheal spaces for lymph node dissection much better that subxiphoid or subcostal VATS (Figure 5). Improved access to the posterior anatomy is a significant advance over the subxiphoid VATS approach, where this is very limited.
- When we perform a left sided uniportal VATS subxiphoid procedure, the instrumentation over the beating heart is one of the most difficult problems during surgery. For this reason in our experience the use of a subcostal approach for the left side may reduce this compression but not avoid it totally. With the robotic platform, this issue seems to be alleviated through the use of CO2 (by pushing the diaphragm and heart down and increasing the space, especially in the left cavity) and the extreme maneuverability of the robot, which seems to diminish the compression on the heart. The cases performed in the cadaver lab showed better exposure on the left side compared with the VATS subcostal or subxiphoid approach. However, we will need to determine if this translates in real patients.
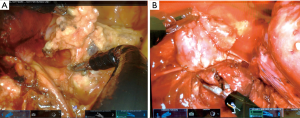
We still find several disadvantages in the SP system that we expect to overcome in the future after clinical trials and further procedure development.
- It is anticipated that the initial cost will be higher compared with the conventional robotic platform, although this is not well detailed since the SP system is not widely available at the time of this writing.
- The SP instrumentation is initially designed primarily for dissection, and robotic staplers for resection are not yet incorporated into the system. We expect robotic staplers will eventually to be included into the platform. However, at this time, all stapling would be performed with a hand-held stapler by the bedside assistant through an additional trocar placed parallel to SP robotic trocar within the same subxiphoid incision. The main surgeon is seated at the console, away from the patient so the assistant must be experienced with VATS stapling as well as familiar with the subxiphoid technique. This is similar to the requirements of the older robotic platforms such as the da Vinci S or Si, for which all stapling was done by the bedside assistant. We recommend to use curve tip staplers for the subxiphoid or subcostal approach to facilitate passage of the tip around vascular structures. However, contrary to the uniportal VATS technique, the staplers are normally inserted with no angulation for most structures bilaterally, due to the oblique direction and low position of the subxiphoid and subcostal space.
- In case of bleeding a thoracoscopic suction can be inserted through the Gel Point access port. However, with CO2 insufflation and a closed chest, the surgeon and assistant will need to be careful not to suction aggressively and cause the lung to re-expand and lose visibility. For VATS subxiphoid or subcostal lung resections, we routine mark an additional thoracic incision before starting the case, at the level of 4th or 5th intercostal space, especially when the case is expected to be not easy in case direct control is needed quickly. This is also the reason for positioning lateral decubitus for lung resections from a subxiphoid or subcostal approach. As during thoracoscopic subxiphoid or subcostal cases, when bleeding is encountered, compression must be the first step. As with conventional robotic surgery, a cigar sponge should also be inserted into the chest cavity at the beginning of the procedure for ready availability. If the bleeding cannot be controlled by subxiphoid approach, a 2–3 cm thoracic incision can be performed to control it through the chest or in combination to both incisions (double port technique). If conversion to open surgery is needed the thoracic incision should be enlarged to a lateral thoracotomy.
- The learning curve for the surgeon using the SP system could be difficult if the surgeon is not familiar with the existing da Vinci multiport robotic platforms. During the learning curve, the movements of the instruments could interfere with one another during some parts of the procedure. However, once the technique is correctly adopted the interference is avoided and the instrumentation is fluid and ergonomic. Camera control to maximize the full capability of the articulation also requires a learning curve, even for existing robotic surgeons.
- The lack of tactile feedback in robotic systems could be a disadvantage especially during the learning curve. We think this issue will not be a problem for the majority of thoracic surgeons due to visual haptics, or the ability to sense tissue tension based on visual cues.
Subcostal SP uniportal robotic-assisted lobectomy
During the subcostal VATS thoracic approach, the lower lobectomies (especially the left side) and left sided resections and anatomic posterior segmentectomies are more difficult. In contrast, in the experimental cadaver labs with the SP system, we found that lower lobes and left side resections are not more difficult than the other locations due to the more curved movements of the instruments and articulated camera combined with the CO2 insufflation, which pushes the diaphragm and mediastinum out of the way (Figures 6,7).
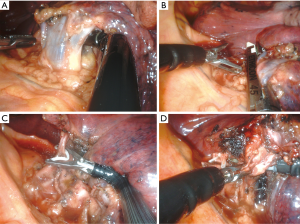
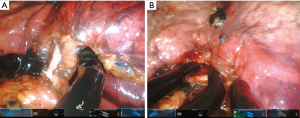
The sequence of dissection for vessels during robotic subcostal lobectomies should be similar to conventional intercostal robotic or subcostal VATS techniques, and ultimately depends on the surgeons’ preference and completeness of the fissure. For right upper lobes we normally prefer to divide first the apical-anterior trunk of the artery and then the upper vein (Figure 8). For left upper lobectomies, the division of the upper lobe vein exposes the artery more clearly (Figure 6). If the fissure is incomplete, a fissureless lobectomy is performed either for upper or lower lobes (Figure 8). For lower lobes if the artery is exposed in the fissure we start with this dissection (Figure 7B).
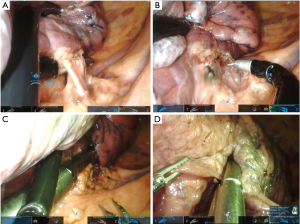
The middle lobe is probably the easiest lobe to start with the subcostal technique and we normally follow the same sequence: vein, anterior part of major fissure, bronchus, artery and minor fissure last.
Compared with the VATS technique, there are distinct differences in instrumentation with the SP platform. For the SP robotic platform there is no right-angle clamp for dissection, ultrasonic shears, or robotic suction. The use of a wristed instrument with 7 degrees of freedom offsets the need for a right-angle clamp, and bipolar energy is the primary energy mode used instead of ultrasonic shears for dissection. There is an SP clip applier for vascular polymer clips that has a wristed design to achieve optimal angles for small vessels through the subcostal view (Figure 6C).
We are very confident that improvements in technology with the development of new SP instruments and experience with the system will overcome these disadvantages in a near future. As surgical experience grows, we believe the indications for SP robotic lobectomy will be extended to include an increasing number of procedures including complex resections. In the future, the implementation of SP robotic staplers will allow the surgeon at the console to perform alone the whole anatomic resection, which may increase the efficiency of the operation.
Despite having proven the feasibility of performing lobectomies in the cadaveric model during experimental labs, evaluation in the clinical setting is mandatory to demonstrate feasibility and safety.
Subxiphoid uniportal SP robotic-assisted thymectomy
The subxiphoid uniportal VATS approach is the ideal technique to perform thymic resections due to bilateral exposure of both pleural cavities, both phrenic nerves, and less postoperative pain (11). However, the VATS technique is not easy because instrumentation is more difficult as the area of dissection is further away, especially for the bilateral upper horn dissection at the thoracic inlet. The use of CO2 could improve the view and exposure by increasing the distance of sternum to mediastinum in more than 1 cm (12). Recently a combination of robotic thoracic surgery and subxiphoid incision was also described (13). The use of sternal retractor is also very helpful to increase the intrathoracic space and improve exposure. Some groups have added an additional small neck incision for a double retraction with a hook on the manubrium, and other groups have added bilateral thoracic or subcostal incisions (14).
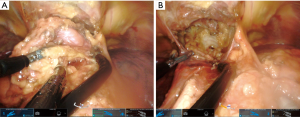
This da Vinci SP robotic system could improve on the VATS approach for subxiphoid thymectomy due to the more versatile instrumentation and visualization. This could facilitate more extended thymectomies and larger anterior mediastinal masses compared with VATS techniques. We have found the cadaver labs to be very promising. As in VATS, the patient is placed in a supine position and we recommend removing the xiphoid process to have more space for the trocar and reduce compression on the heart (Figure 9). Thanks to the subxiphoid approach we can expose both cavities by opening both pleuras and expose both phrenic nerves (Figure 10A). The use of CO2 at 6–8 mmHg in combination with an optional sternal retractor clearly improves the visualization. In addition, thanks to the use of wristed instruments with more freedom of movement, the upper thymic horns are easily removed compared with other single incision techniques with visualization well into the lower neck from the subxiphoid access (Figure 10B). Moreover, we believe we can achieve a radical resection of thymus with no need for a cervical incision for sternal retraction.
Conclusions
The da Vinsi SP robotic platform has been evaluated in the experimental lab setting on cadavers for both subcostal lobectomy and subxiphoid thymectomy with promising exposure and workflow. The visualization and ability to perform a precise dissection appears significantly improved over the existing VATS approaches. Further studies in the clinical setting will be necessary to prove feasiblity and safety, but this appears to be the future platform for thoracic approaches due to the lack of an intercostal incision and less pain.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors of this chapter have received compensation from Intuitive Surgical for consulting and/or educational services. The experimental labs discussed were supported by Intuitive Surgical.
References
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Gonzalez-Rivas D, Sihoe AD. Important Technical Details During Uniportal Video-Assisted Thoracoscopic Major Resections. Thorac Surg Clin 2017;27:357-72. [Crossref] [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxiphoid single-incision thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg 2014;148:3250-1. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Gonzalez-Rivas D, Lirio F, Sesma J, et al. Subxiphoid complex uniportal video-assisted major pulmonary resections. J Vis Surg 2017;3:93. [Crossref] [PubMed]
- Guido Guerrero W, Hernandez Arenas LA, Jiang G, et al. Subxiphoid mediastinal lymphadenectomy. J Vis Surg 2016;2:105. [Crossref] [PubMed]
- Ali J, Haiyang F, Aresu G, et al. Uniportal Subxiphoid Video-Assisted Thoracoscopic Anatomical Segmentectomy: Technique and Results. Ann Thorac Surg 2018;106:1519-24. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-assisted, video-assisted thoracoscopic and open lobectomy: Propensity-matched analysis of recent Premier data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Wu L, Lin L, Liu M, et al. Subxiphoid uniportal thoracoscopic extended thymectomy. J Thorac Dis 2015;7:1658-60. [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-8. [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Zielinski M, Czajkowski W, Gwozdz P, et al. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg 2013;44:e113-9; discussion e119.


