
Pattern of recurrence after CyberKnife stereotactic body radiotherapy for peripheral early non-small cell lung cancer
Introduction
Stereotactic body radiotherapy (SBRT) allows for the application of large doses of radiation to the tumor with minimal exposure to the surrounding organs. Its methods have been gradually trialed for lung cancer in the past 20 years, with increased stability and precision and the development of related technologies such as image-guided navigation. SBRT is now considered a treatment option for patients with stage I non-small cell lung cancer (NSCLC) with inoperable status or those who refuse surgery, based on phase II trials (1,2). The results of a phase II trial for operable patients and a phase III randomized study comparing SBRT with surgery have recently been published (3,4). The indications for SBRT have been broadened to include operable patients.
Previous reports described predictive factors of local control (LC) after SBRT. Biological effective dose (BED) and tumor diameter were predominantly regarded as predictive factors of local recurrence (5). CyberKnife deliver radiation dose from numerous angles with real-time tumor tracking which corrects for tumor motion during respiration by repositioning the radiation beam to track the moving tumor. So, the dose distributions and safety margin differ between CyberKnife SBRT and conventional SBRT. The treatment efficacy after CyberKnife SBRT have not been adequately addressed.
Concerns remain about the risk of nodal and distant recurrence after SBRT. There were several reports on nodal recurrence. It was mentioned that incidental hilar dose greater than 20 Gy reduced ipsilateral hilar relapse (6). However, recurrence pattern after SBRT is versatile, involved lobe alone, involved lobe and disseminated, lymph node alone, lymph node and disseminated, and disseminated alone (2). Predictive factors of recurrence outside the irradiation field are unknown.
The purpose of this study was to investigate pattern of recurrence according to irradiation field after CyberKnife SBRT for peripheral early stage NSCLC.
Methods
Patients
This retrospective study included patients with peripheral cT1/2N0M0 NSCLC that was treated with SBRT using a CyberKnife VSI System (Accuray Inc., Sunnyvale, CA, USA) between May 2013 and March 2016 at Kobe Minimally invasive Cancer Center and followed up by more than two imaging examinations. Both operable and inoperable patients were included. Peripheral lesion was defined as a tumor more than 2 cm from all directions of any mediastinal critical structure, including the bronchial tree, esophagus, heart, brachial plexus, major vessels, spinal cord, phrenic nerve, and recurrent laryngeal nerve. Patients with double lung tumors and other synchronous malignancies were excluded. Tumor histology was proved by a transthoracic or bronchoscopic biopsy. In instances where histology could not be proved, patients were treated when the tumor was growing temporally and diagnosed with clinical lung cancer by a pulmonologist and radiologist. Clinical staging of lung cancer was performed according to 8th Union for International Cancer Control TNM staging system using computed tomography (CT), brain magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose positron emission tomography/CT.
Treatment
The CyberKnife SBRT method for lung cancer was performed as previously described (7). Image guidance is performed by two orthogonal X-ray images taken during breathing. The spine, directed tumor, and fiducial tracking systems were used properly. In the fiducial tracking system, the intravascular method was used to place a single fiducial marker close to the tumor. The patients were immobilized with a Vac-Lok cushion (CIVCO, USA). A thin-sliced 4-dimensional CT scan without contrast enhancement was recorded with 1-mm slices. The organs at risk (i.e., the spinal cord, normal lung tissue, heart, and esophagus) were contoured on the CT scan in the resting respiratory level. Gross tumor volumes (GTVs) were contoured on each phase of the 4-dimensional CT scan registered with the fiducial marker in the fiducial tracking system, the tumor itself in the tumor tracking system, and the vertebral body in the spine tracking system. The internal target volume was defined as the fusion of all GTVs for each phase of the 4-dimensional CT scan. The planning target volume (PTV) was defined as the internal target volume plus 2–6 mm. This range of PTV margin was resulting from recognition accuracy of spine, tumor or fiducial marker in demonstration. Treatments were planned using MultiPlan 4.6.0 treatment planning software (Accuracy Inc., Sunnydale, CA, USA). Radiation doses were calculated using the Monte Carlo algorithm. The treatments consisted of a 6 MV radiation beam using one or two circular collimator cones. The prescribed radiation doses were 75–85% the isodose line of the PTV, covering ≥95% of the volume in 4 equal fractions. However, a PTV under-dosage was permitted in order to protect the organ at risk. Adjuvant systemic therapy was not permitted.
Follow-up
After the treatment, CT was performed every 3 months during the first year and at 6 months intervals thereafter. MRI of the brain was performed once a year or when clinical signs and symptoms suspicious for brain involvement were present. Progression-free survival (PFS) was defined as the time from the date of the start of SBRT until the earliest signs of disease progression or death from any cause. Overall survival (OS) was defined as the time from the date of SBRT until the date of death from any cause and was censored at the date of the last follow-up for surviving patients. Infield recurrence was defined as the evidence of tumor volume enlargement or the appearance of a new lesion in the PTV. Suspected recurrence was confirmed by at least two imaging modalities, with final diagnosis by histology. If the histology findings were inconclusive or not available, recurrence was diagnosed by the agreement of more than 2 physicians. Other new lesions were classified into out-of-field tumor progression. Toxicity was graded according to the Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analyses
All statistical analyses were conducted using R software, version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria). PFS and OS curves were estimated using the Kaplan-Meier product-limit method with 95% confidence intervals (CI). Cumulative incidence curves of recurrence were calculated and compared using the Gray’s test. The Gray’s test was performed using the following factors, age, gender, Eastern Cooperative Oncology Group performance status score, pack-years, operability, forced expiratory volume in 1 second as percent of forced vital capacity, vital capacity as percent of predicted, histology, epidermal growth factor receptor (EGFR) status, T stage, GTV, dose to 95% of the PTV (PTV D95) and diagnosis-to-treatment intervals (DTI). All tests were two-sided. P values <0.05 were considered statistically significant.
Results
After excluding those with double lung tumors (n=2) and synchronous uncontrolled malignancies (n=1), 71 patients with peripheral NSCLC treated using the CyberKnife System were included in this study. The median follow-up period for surviving patients was 34 months (range, 7–64 months). The patient characteristics and treatment parameters are summarized in Table 1. Median age was 78 years (range, 57–90 years). None of the patients had a PS >2. There were 19 patients whose tumor was not histologically proven. Five adenocarcinoma patients had EGFR mutation. Among them, only one patient had history of lung cancer. Median prescription dose was 56 Gy (range, 48–60 Gy) in 4 equal fractions. Marker placement was enforced for 36 patients. The spine, directed tumor, and fiducial tracking systems were used in 11, 24, and 36 patients, respectively.
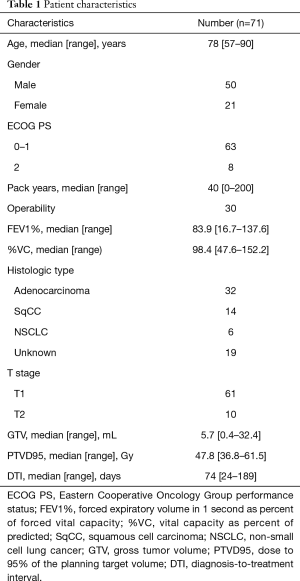
Full table
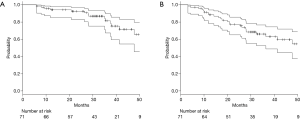
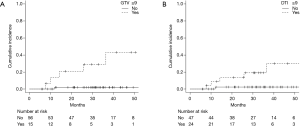
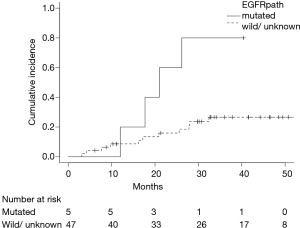
Fourteen (20%) patients died during follow-up. Six deaths were related to progression of lung cancer and another 8 were related to other disease. The 2-year OS rate was 93% (95% CI: 83–97%) (Figure 1A). During follow-up period, 20 patients (28%) had recurrences and the 2-year PFS rates were 77% (95% CI: 65–86%) (Figure 1B). Infield and out-of-field recurrence were observed in 6 and 16 (regional node only, 7; distant only, 6; and both node and distant, 3), respectively. Two of them had synchronous infield and out-of-field recurrences. Two-year cumulative incidence rate of infield recurrence and out-of-field recurrence were 6% (95% CI: 2–14%) and 17% (95% CI: 9–27%), respectively. Predictive factors of infield and out-of-field recurrence were analyzed by Gray’s test (Table 2). Fifteen patients had GTV ≥9 mL, and GTV ≥9 mL was significantly associated with infield recurrence (P<0.001) (Figure 2A). Twenty-four patients had DTI ≥90 days, and GTV ≥90 days was also significantly associated with infield recurrence (P=0.007) (Figure 2B). Among 52 histologically proven NSCLC patients, EGFR mutation was significantly associated with out-of-field recurrence (P=0.014) (Figure 3).
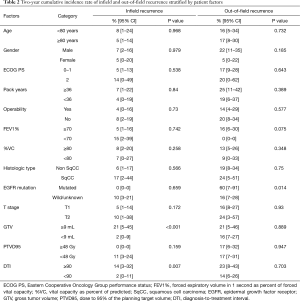
Full table
A grade 3 adverse event (radiation pneumonitis) was observed in 1 case (1%). Grade 2 adverse events (7 instances of radiation pneumonitis, 4 of rib fractures and 1 of brachial plexopathy) were observed in 13 patients (18%). Grade 1 adverse events (57 instances of radiation pneumonitis, 6 of cough, and 2 of chest pain) were observed in 65 patients (92%). There were no grade 4 or 5 events. All 36 fiducial markers were placed using an intravascular approach. Regarding toxicities related to the method of fiducial marker placement, only 1 patient (3%) was diagnosed with a grade 1 femoral hematoma. No coil migration was observed.
Discussion
This retrospective study investigated and analyzed 71 patients with peripheral stage I NSCLC treated with SBRT using a CyberKnife system. Two-year cumulative incidence rate of infield recurrence and out-of-field recurrence were 6% and 17%, respectively. GTV ≥9 mL was significantly associated with infield recurrence (P<0.001), and EGFR mutation was significantly associated with out-of-field recurrence (P=0.014). To the best of our knowledge, this is a first study to demonstrate the clinical significance of EGFR mutation in patients with early-stage NSCLC who received SBRT although EGFR mutated patients were observed in only 5.
Nagata et al. reported 3-year LC rates for inoperable and operable populations of 87% and 85%, respectively (3). Timmerman et al. reported a 3-year LC rate of 98% (2) for inoperable populations and a 4-year rate of 96% for operable populations (8). These rates of prospective studies are identical to our present study. The predictive factors of LC were previously discussed. Onishi et al. described improved LC in 257 patients with BED10 ≥100 Gy compared to BED10 <100 Gy (9). Grills et al. observed that BED10 ≥105 Gy was related to good LC (10). In our study, PTV D95 ≥48 Gy (approximately equal to BED10 105 Gy) was not a significant factor for good infield control (P=0.159). Onimaru et al. (11) and Davis et al. (12) reported that higher prescription doses improved LC in T2 but not in T1 tumors. T2 stage was not associated with infield control in our study, but GTV ≥9 mL was significantly associated (P<0.001). Furthermore, TDI ≥90 days was significant predictive factor of infield recurrence. It has been described also delay in treatment initiation may affect outcome (13). In case of using fiducial tracking, it is possible that the treatment initiation may be delayed as compared to other tracking methods because marker placement is necessary. Suggesting that it is necessary to start early treatment from diagnosis taking that delay into consideration. In consideration of that delay, it is necessary to start treatment as soon as possible from diagnosis.
In our study, out-of-field recurrence was seen in 16 patients and 2-year cumulative incidence was 17%. Previous reports showed the 2-year cumulative incidence of nodal and distant recurrences were 9.0–16.1%, and 14.6–15.5%, respectively (14,15). They found no variables associated with regional recurrence, and tumor size was an independently significant predictor for distant recurrence. In this study, tumor size was not a predictive factor of out-of-field recurrence, but EGFR mutation was significantly associated with higher out-of-field recurrence (P=0.014). The findings of driver mutations, such as EGFR mutation and anaplastic lymphoma kinase translocation, brought paradigm shifts in the therapeutic strategy for advanced NSCLC (16,17). However, there have been some retrospective reports regarding the role of EGFR mutation on recurrence pattern in locally advanced NSCLC patients who received chemoradiotherapy (18-21). The reports found that EGFR mutation is a positive factor for infield control, and that patients harboring this mutation are at high risk of out-of-field relapse. There was no statistically significant difference, but 2-year cumulative incidence of infield recurrence was 0% in this study. Therefore, similar pattern of recurrence in EGFR mutated tumor may be expected in early stage NSCLC.
We observed 1 instance of grade 3, 7 instances of grade 2 radiation pneumonitis, and no grade 4 or 5 incidents. These rates are almost identical to those of previous reports (22). Among adverse events relating to fiducial marker placement, only 1 grade 1 event and no migration were observed. Adverse events relating fiducial marker placement should also be evaluated for CyberKnife SBRT. Our results indicate that the intravascular fiducial marker placement method is a safe and more reliable option.
SBRT is a treatment that is minimally invasive and good treatment results can be expected. On ASTRO guideline, SBRT for standard operable patients is not recommended outside of a clinical trial (13). Now, some comparative randomized studies (NCT01753414, NCT02629458, NCT02468024, NCT02984761) are ongoing (23). In order for SBRT to be a parallel option for operable patients, the results of those clinical trials are desirable.
Our study has several limitations. First, the use of a retrospective design means that our findings may be prone to selection bias. Second, the total number of events in our cohort was relatively small. Third, evaluation of OS was difficult since our follow-up period was relatively short at 34 months. However, Senthi et al. described the median times to local, regional, and distant recurrence were 14.9, 13.1, and 9.6 months, respectively (24). So, evaluation of recurrence is considered possible.
In conclusion, the incidence of recurrence after SBRT for peripheral cT1/2N0M0 NSCLC was similar to those reported in the literature and GTV was the significant predictive factor of infield recurrence. We are the first to report that EGFR mutation is related with out-of-field recurrence after SBRT for early-stage lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Research Ethics Committee of Kobe Minimally invasive Cancer Center [reference number: 2016-(kenkyu05)-03] and written informed consent was obtained from all patients. The research was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
References
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Ohri N, Werner-Wasik M, Grills IS, et al. Modeling local control after hypofractionated stereotactic body radiation therapy for stage I non-small cell lung cancer: a report from the elekta collaborative lung research group. Int J Radiat Oncol Biol Phys 2012;84:e379-84. [Crossref] [PubMed]
- Lao L, Hope AJ, Maganti M, et al. Incidental prophylactic nodal irradiation and patterns of nodal relapse in inoperable early stage NSCLC patients treated with SBRT: a case-matched analysis. Int J Radiat Oncol Biol Phys 2014;90:209-15. [Crossref] [PubMed]
- Nakamura M, Nishimura H, Nakayama M, et al. Dosimetric factors predicting radiation pneumonitis after CyberKnife stereotactic body radiotherapy for peripheral lung cancer. Br J Radiol 2016;89:20160560. [Crossref] [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol 2018;4:1263-6. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol 2012;7:1382-93. [Crossref] [PubMed]
- Onimaru R, Fujino M, Yamazaki K, et al. Steep dose-response relationship for stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:374-81. [Crossref] [PubMed]
- Davis JN, Medbery C 3rd, Sharma S, et al. Stereotactic body radiotherapy for early-stage non-small cell lung cancer: clinical outcomes from a National Patient Registry. J Radiat Oncol 2015;4:55-63. [Crossref] [PubMed]
- Loganadane G, Martinetti F, Mercier O, et al. Stereotactic ablative radiotherapy for early stage non-small cell lung cancer: A critical literature review of predictive factors of relapse. Cancer Treat Rev 2016;50:240-6. [Crossref] [PubMed]
- Spratt DE, Wu AJ, Adeseye V, et al. Recurrence Patterns and Second Primary Lung Cancers After Stereotactic Body Radiation Therapy for Early-Stage Non-Small-Cell Lung Cancer: Implications for Surveillance. Clin Lung Cancer 2016;17:177-83.e2. [Crossref] [PubMed]
- Giuliani ME, Hope A, Mangona V, et al. Predictors and Patterns of Regional Recurrence Following Lung SBRT: A Report From the Elekta Lung Research Group. Clin Lung Cancer 2017;18:162-8. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic Lymphoma Kinase Inhibition in Non-Small-Cell Lung Cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Akamatsu H, Kaira K, Murakami H, et al. The impact of clinical outcomes according to EGFR mutation status in patients with locally advanced lung adenocarcinoma who recieved concurrent chemoradiotherapy. Am J Clin Oncol 2014;37:144-7. [Crossref] [PubMed]
- Tanaka K, Hida T, Oya Y, et al. EGFR Mutation Impact on Definitive Concurrent Chemoradiation Therapy for Inoperable Stage III Adenocarcinoma. J Thorac Oncol 2015;10:1720-5. [Crossref] [PubMed]
- Yagishita S, Horinouchi H, Katsui Taniyama T, et al. Epidermal growth factor receptor mutation is associated with longer local control after definitive chemoradiotherapy in patients with stage III nonsquamous non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2015;91:140-8. [Crossref] [PubMed]
- Lim YJ, Chang JH, Kim HJ, et al. Superior Treatment Response and In-field Tumor Control in Epidermal Growth Factor Receptor-mutant Genotype of Stage III Nonsquamous Non-Small cell Lung Cancer Undergoing Definitive Concurrent Chemoradiotherapy. Clin Lung Cancer 2017;18:e169-78. [Crossref] [PubMed]
- Baker R, Han G, Sarangkasiri S, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys 2013;85:190-5. [Crossref] [PubMed]
- Levy A, Mercier O, Le Pechoux C. Stereotactic ablative body radiation therapy or surgery for operable early non-small cell lung cancer patients: bound hand and foot to evidence. J Thorac Dis 2017;9:482-4. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJA, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]


