
Impact of volume reduction in giant left atrium during surgical ablation of atrial fibrillation
Introduction
In the presence of atrial fibrillation (AF), a giant left atrium (LA) is known to further contribute to hemodynamic and respiratory complications, as well as to enhanced risk of thrombi formation (1). In addition, the enlarged LA itself is a well-known risk factor for AF recurrence after the surgical AF ablation (2).
In order to improve the outcomes of surgical AF ablation in the setting of enlarged LA, various techniques of LA reduction surgery have been introduced (1-11), and have shown improved sinus rhythm restoration rates (2,6,9,10). Despite these promising results of LA reduction procedures, however, there has been no clear consensus on whether to perform the surgical AF ablation procedure in patients with a giant LA, and whether to perform LA reduction in addition to surgical AF ablation in the presence of giant LA during mitral valve (MV) surgery (12). These controversies mainly stem from the lack of robust evidences such as results from randomized trials, but are also partly attributable to the heterogeneity of AF ablation/LA reduction procedures and their mixed results. For instance, techniques of LA suture plication and partial resection, the most common types of LA reduction, have been criticized that they may be less effective in reducing LA volume (12). More aggressive form of circumferential LA resection (CLAR) as a method for LA reduction is known to more effectively reduce the LA volume (3,4,11,13), but it has not been widely used because of concerns on risk of bleeding and longer procedural time. In the belief that CLAR is the most effective LA reduction method, and is reproducible with acceptable procedural safety, we have endeavored on this procedure in patients with giant LA associated AF. This study aims to evaluate the impact of CLAR as a LA reduction procedure on surgical outcomes of AF ablation in patients with a giant LA.
Methods
Patients
This study was approved by the Institutional Review Board and Ethics committee and the requirement for individual patient consent was waived.
Between January 2000 and December 2011, 116 patients with a giant LA, who underwent the surgical AF ablation concomitantly with MV surgery, were retrospectively reviewed. A giant LA was defined as having a LA antero-posterior dimension, larger than 70 mm on the M-mode echocardiogram at the end systolic phase. Among these, 28 patients received CLAR procedure (reduction group) while the other 88 patients received the surgery without LA reduction (non-reduction group).
The decision to perform CLAR procedure was influenced by LA size and individual patients’ baseline risk. Based on not only antero-posterior dimension of LA but on the 3-dimensional size of LA, we decided to perform CLAR in patients with a large LA, not to leave a substantial substrate for maintaining AF. However, patients who would be adversely affected by the prolonged operation time, esp. elderly over 70 years older, patients with LV dysfunction or severe co-morbidity were not candidates of the CLAR procedure. The final decision was made by the attending surgeon’s discretion.
Operative techniques
Detailed operative techniques and schematic illustrations of CLAR procedure were described in our previous article (11). After a median sternotomy, patients were placed on a standard cardiopulmonary bypass (CPB) with bicaval cannulation and cooled to 32 °C. For surgical exposure, cannulation of the superior vena cava (SVC) was performed at a site as high as possible. Cardiac arrest was induced with blood cardioplegic solution through antegrade and/or retrograde cannula. Whether to perform an SVC transection was determined by the degree of the surgical exposure. SVC transection was needed only in 29% of reduction group. A standard right-sided left atriotomy was made parallel to the interatrial groove. The inferior end of the left atriotomy was extended, aiming it to the left inferior pulmonary vein. The other end of the left atriotomy was extended superiorly across the LA dome, and this finally met the inferior extension of the atriotomy. After making another incision, parallel to the mitral annulus leaving about a 2 cm margin from the annulus, a strip with a width of 2–4 cm was resected circumferentially, and this included the LA appendage (Figure 1). Following the circumferential resection of the LA wall, left isthmic ablation was performed using cryoablation (Frigitronics, Cooper Surgical, Shelton, CT, USA). Thereafter, MV surgery was performed. The LA was then closed with 4-0 prolene continuous sutures. A right atriotomy was made and a right-sided AF ablation was performed (Figure 2A). Concomitant procedures and the remaining surgery were performed as per the usual standards.

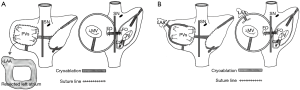
For the surgical ablation for AF in the patients of non-reduction group, LA incision was made. The LA ablation was done endocardially before the MV procedure, which included a pulmonary veins isolation line, a line from the pulmonary isolation lesion to the LA appendage, and another line from the pulmonary isolation lesion to the MV annulus, posteriorly (Figure 2B). The right atrial ablation was performed through an oblique right atriotomy. Endocardial ablation included cavotricuspid isthmus lesion, a line from the SVC to inferior vena cava (IVC), and a line from the superior end of atriotomy to the anterior tricuspid annulus.
Postoperative protocol
Anticoagulation therapy was started on the day after the surgery, upon confirmation of cessation of bleeding. In patients with tissue valve replacement or MV repair, anticoagulation was discontinued after 3 months if the normal sinus rhythm was restored, and patients were switched to aspirin. Amiodarone was used in all of the patients for about 3 months, unless it was contraindicated.
Follow-up electrocardiograms (ECGs) after discharge were repeatedly performed at 3- to 6-month interval in the outpatient clinic. In patients with palpitations or symptoms related to atrial arrhythmias beyond the initial blanking period, 24-hour Holter monitoring was performed. However, we could not perform Holter monitoring for all patients in routine basis due to non-compliance of many patients from remote areas and to economical reason.
Definition and follow-up
Recurrent AF was defined as any AF, atrial flutter or atrial tachycardia of at least 30 seconds that is documented by ECGs or rhythm recording system after the 3 months blanking period. Follow-up data were acquired through clinical examination and/or telephone interviews and follow-up was closed on February 28, 2014. The follow-up data were 90.0% complete at the mean of 6.8±3.0 years (1.7–13.8). The total follow-up period was 674.5 patient-years. Cardiac rhythm follow-up for 12 months or later was possible in 96 patients (87.3%) at the mean of 5.9±2.8 (1.0–13.0). Survival data were 100% complete by using the data from the Korean National Health Insurance Corporation.
Statistical analysis
Continuous variables are expressed as the means ± standard deviations, and were compared with the Mann-Whitney U test. Categorical data are expressed as counts and proportions, and were compared with Fisher’s exact test. Kaplan-Meier method was used to delineate freedom from AF, and log-rank test was used to compare the rates between groups. For multivariable analysis to determine independent risk factors of AF recurrence, Cox-proportional hazard models were used. For this, variables with P<0.2 on univariable Cox-hazard models were selected to enter multivariable models. Parameters of LA volume and dimensions between preoperative and postoperative periods were compared with Wilcoxon signed-rank test. A significant difference between the measurements was defined at P values of 0.05 or less. All data analyses were performed with SPSS software version 22 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
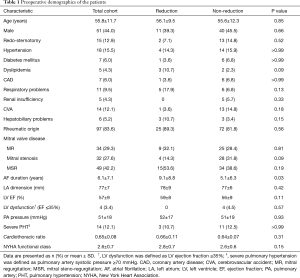
Preoperative baseline profiles of the patients were listed on Table 1. Most patients (83.6%) included in this study had rhematic etiology and age tended to be relatively young. All patients presented with persistent AF or longstanding persistent AF.
Full table
Most of the preoperative characteristics in the two groups did not significantly differ except for the AF duration, which was significantly longer in reduction group than non-reduction group (9.1±8.8 vs. 5.1±6.3 years, P=0.03).
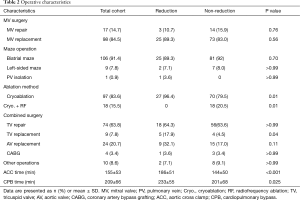
Operative characteristics are summarized in Table 2. MV repair was performed in 14.7% (n=17). In patients with MV replacement (84.5%, n=98), mechanical valve was used in 67 patients. For AF ablation, biatrial maze operation was conducted for majority of patients (91.4%, n=106), while left-sided maze procedure (7.8%, n=9) and pulmonary vein isolation (0.9%, n=1) were used in some. As an energy source for ablation, cryoablation was used exclusively in reduction group and most of non-reduction group. Only 20.5% (n=18) in non-reduction group underwent ablation using radiofrequency ablation combined with cryoablation.
Full table
Aortic cross clamp (ACC) and CPB times were longer in reduction group than non-reduction group (ACC: 186±51 vs. 144±50 min, P<0.001; CPB: 233±55 vs. 201±68 min, P=0.025).
Perioperative results
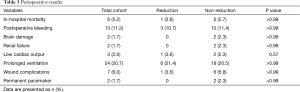
Table 3 shows the summary of perioperative results. In-hospital mortality occurred in 6 (5.2%) patients. The causes of deaths were right heart failure in 2 patients, biventricular failure in 1 patient, respiratory failure in 2 patients, and brain hemorrhage in 1 patient. With respect to rates of early mortality and morbidity, there were no significant differences between the two groups.
Full table
Follow-up results
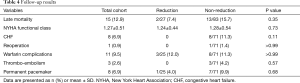
The follow-up results are listed on Table 4. During the follow-up period, late death occurred in 15 (12.9%) patients without a significant inter-group difference. Congestive heart failure occurred only in non-reduction group (n=8), but the difference in the rates was not statistically significant (P=0.11). Patients with a mechanical valve and AF-recurred patients needed anticoagulation. In terms of warfarin-related complications and thrombo-embolism, there was no difference between the two groups. Total 8 patients (6.9%), including 2 peri-operative pacemaker cases, needed permanent pacemaker insertion without difference between the two groups.
Full table
AF recurrence and risk factors
Reduction group showed superior freedom rates from AF than non-reduction group throughout the whole study period. In reduction group, the freedom rates from AF were 84.2%, 74.3%, and 54.5% at 1, 3 and 5 years, respectively, compared with 49.0%, 33.2%, and 28.4%, respectively in non-reduction group (P=0.013) (Figure 3). At the last visit, only two patients (one patient of each group) in sinus rhythm were taken antiarrhythmic agents.
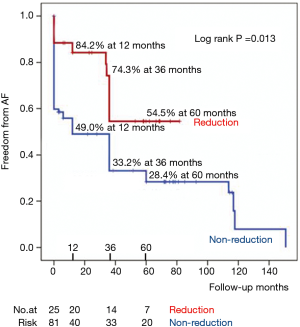
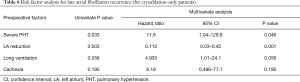
Table 5 shows results of univariable and multivariable analyses of risk factors for AF recurrence. Multivariable analysis revealed severe pulmonary hypertension as an independent risk factor for the recurrence of AF. Meanwhile, LA reduction and cryoablation were the protective factors for the recurrence of AF.

Full table
To remove the confounding effect of different energy sources, we analyzed risk factors for AF recurrence in patients who underwent ablation using cryoablation exclusively (Table 6). The result also confirmed LA reduction as a strong protective factor for the recurrence of AF.
Full table
LA dimension and volume changes
A follow-up echocardiographic data was available in 108 patients at the mean duration of 34.7±32.8 months (range, 0.2–112.0 months). There was no recurrent mitral regurgitation (more than grade II) in 17 patients who underwent MV repair. LA antero-posterior dimension was more significantly reduced to 54±10 mm in reduction group comparing with 64±10 mm in non-reduction group (P<0.001).
To check the reduction effect of CLAR, LA volume analysis was performed in patients who had stored echocardiographic images both the preoperative baseline and postoperative. Twenty patients of reduction group and 48 patients of non-reduction group were included for the early postoperative (within 30 days) assessments. LA volume was significantly reduced from 355±138 to 181±65 mL in reduction group and from 372±164 to 261±99 mL in non-reduction group (P=0.01).
For the late follow-up assessment, 17 patients in reduction group and 32 patients in non-reduction group were available (38.1±7.8 months, range 27.1–47.3 months). The last LA volume was 141±41 mL in reduction group and 256±111 mL in non-reduction group (P<0.001). LA volume reduction was more significant in reduction group (57% reduction) than in non-reduction group (29% reduction) (P<0.001).
Discussion
In this study, we demonstrated that (I) CLAR in addition to AF ablation is a safe procedure without affecting the perioperative mortality and morbidity despite the increase of ACC and CPB times; (II) LA volume reduction of CLAR was effective with the mean reduction rate of 57%; and (III) aggressive LA volume reduction showed superior freedom rates from AF than non-reduction group, throughout the whole study period.
Cardiac auto-transplantation for LA volume reduction, introduced by Batista et al. (14), was modified to the “partial auto-transplantation technique”, which transect the aorta, the pulmonary artery and the SVC while preserving the IVC (3). It was further modified later to the technique which cut only the SVC for the procedure (15). CLAR is a definitive surgical goal of cardiac auto-transplantation for LA reduction in patients with a giant LA. According to our experience, the procedure was performed even without cutting the SVC in more than 70% of the patients.
Regarding on the safety issue of CLAR, major concerns were the prolonged ACC and CPB times, and surgical bleeding (12). In this comparative study, an additional 30 to 40 minutes of ACC and CPB times did not translate into an increase in postoperative mortality and morbidities. García-Villareal et al. (16) demonstrated a slight prolongation of ACC and CPB times in the partial auto-transplantation group compared with the patients without (134±15 and 113±14 minutes versus 116±16 and 99±17 minutes, respectively). However, we don’t recommend to perform CLAR in elderly or comorbid patients, who would be adversely affected by the prolonged ACC and CPB times. With respect to surgical bleeding, there was no difference between the two groups.
In terms of LA volume reduction, the reduction effect of CLAR procedure was drastic. Various techniques of LA reduction surgery have been introduced (1-11) such as LA plications or LA partial resections, and these need to be compared with CLAR type procedures desirably in randomized studies to suggest optimal surgical strategy.
Another notable benefit of CLAR was the higher rates of AF freedom than in the control group throughout the whole study period. Even though the preoperative AF duration, one of the strong risk factors of AF recurrence, was longer in reduction group, the freedom rates from AF were superior in the group. The higher AF freedom rates would be attributed to LA volume reduction after CLAR, a protective factor of the recurrence of AF.
The other explanation for the higher AF freedom rates might be the cut & sew effect of CLAR. Although cryoablation and radiofrequency ablation, as alternative methods of cut & sew technique, showed favorable results, the issue of ensuring completely transmural lesions remains unsolved. According to Stulak et al. (17), the Cox maze III procedure, cut & sew technique, was independently associated with less risk of recurrent AF at all follow-up points after surgery compared with alternative energy sources. As an energy source for AF ablation, cryoablation was also found to be a protective factor against the recurrence of AF in this study. However, this result might also be affected by the exclusive use of cryoablation in LA reduction group.
Long-standing persistent/persistent AF with a giant LA is a very difficult condition to treat AF. According to Dr. Kim et al. (18), the AF-free rate at 5 years in the larger LA (≥70 mm) group was 59.4% and significantly lower compared with 72.3% in the smaller LA (<70 mm) group. These increased failure rates of surgical ablation in patients with giant LA may be attributed to the redundant atrial tissue providing enough substrates for the formation of macro-reentry circuit and atrial remodeling including atrial fibrosis, which affects on the conduction velocity and the refractory period (19). So, the definite methods of LA volume reduction like CLAR would be needed to have better outcomes after surgical AF ablation in patients with giant LA (LA dimension ≥70 mm).
In contrast to reduction group, the AF freedom rates of the control group were very poor, showing 33% at 3-year and 28% at 5-year follow-up. This poor result paradoxically indicates that surgical AF ablation without LA volume reduction is inappropriate for the patients with a giant LA and AF.
Through this comparative study, we attempted to assess the effect of LA volume reduction on symptom improvement and congestive heart failure protection. Although it was statistically insignificant, congestive heart failure occurred mainly in the control group, non-reduction group (8 patients, 11.3%) and in none of the patients of reduction group. This tendency seemed to attributable to the improved sinus rhythm maintenance and the decreased AF recurrence in reduction group.
This study is subject to the limitations inherent in retrospective non-randomized study and the relatively small number of patients in reduction group. Data from a larger number of patients with randomized prospective study design might solve these limitations. Also, the results of this study could have been affected by procedural selection bias. But, most of the preoperative characteristics in the two groups did not significantly differ except for the AF duration. Most patients (83.6%) included in this study had rhematic etiology and age tended to be relatively young; therefore, the facts have to consider when interpreting the results of this study. Finally, the detection of AF, based on the routine or spot electrocardiogram obtained during the follow-up visits could have been underestimated the real incidence of AF. However, we could glance the rhythm trends of surgically ablated patients with a giant LA.
Conclusions
In patients with a giant LA, surgical AF ablation alone may not be sufficient to optimize the rhythm results. Aggressive LA volume reductions in this setting may improve the success rates of surgical AF ablation. The study results suggest that CLAR is a safe and effective method of LA volume reductions.
Acknowledgements
We thank Dr. Jun Bum Kim (Department of Thoracic and Cardiovascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul) for his proofreading and writing assistance of this article.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board and Ethics Committee of Sejong General Hospital (IRB /2012-387) and the requirement for individual patient consent was waived.
References
- Kawazoe K, Beppu S, Takahara Y, et al. Surgical treatment of giant left atrium combined with mitral valvular disease. Plication procedure for reduction of compression to the left ventricle, bronchus, and pulmonary parenchyma. J Thorac Cardiovasc Surg 1983;85:885-92. [PubMed]
- Scherer M, Therapidis P, Miskovic A, et al. Left atrial size reduction improves the sinus rhythm conversion rate after radiofrequency ablation for continuous atrial fibrillation in patients undergoing concomitant cardiac surgery. Thorac Cardiovasc Surg 2006;54:34-8. [Crossref] [PubMed]
- Lessana A, Scorsin M, Scheublé C, et al. Effective reduction of a giant left atrium by partial autotransplantation. Ann Thorac Surg 1999;67:1164-5. [Crossref] [PubMed]
- García-Villarreal OA, Rodríguez H, Treviño A, et al. Left atrial reduction and mitral valve surgery: the "functional-anatomic unit" concept. Ann Thorac Surg 2001;71:1044-5. [Crossref] [PubMed]
- Sinatra R, Pulitani I, Antonazzo A, et al. A novel technique for giant left atrium reduction. Eur J Cardiothorac Surg 2001;20:412-4. [Crossref] [PubMed]
- Romano MA, Bach DS, Pagani FD, et al. Atrial reduction plasty Cox maze procedure: extended indications for atrial fibrillation surgery. Ann Thorac Surg. 2004;77:1282-7; discussion 1287. [Crossref] [PubMed]
- Badhwar V, Rovin JD, Davenport G, et al. Left atrial reduction enhances outcomes of modified maze procedure for permanent atrial fibrillation during concomitant mitral surgery. Ann Thorac Surg 2006;82:1758-63; discussion 1764.
- Sugiki H, Murashita T, Yasuda K, et al. Novel technique for volume reduction of giant left atrium: simple and effective "spiral resection" method. Ann Thorac Surg 2006;81:378-80. [Crossref] [PubMed]
- Marui A, Saji Y, Nishina T, et al. Impact of left atrial volume reduction concomitant with atrial fibrillation surgery on left atrial geometry and mechanical function. J Thorac Cardiovasc Surg 2008;135:1297-305. [Crossref] [PubMed]
- Wang W, Buehler D, Martland AM, et al. Left atrial wall tension directly affects the restoration of sinus rhythm after Maze procedure. Eur J Cardiothorac Surg 2011;40:77-82. [Crossref] [PubMed]
- Kim JH, Na CY, Lee SJ, et al. Circumferential left atrium resection for treating a giant left atrium. J Card Surg 2013;28:102-8. [Crossref] [PubMed]
- Apostolakis E, Shuhaiber JH. The surgical management of giant left atrium. Eur J Cardiothorac Surg 2008;33:182-90. [Crossref] [PubMed]
- Troise G, Cirillo M, Brunelli F, et al. Mid-term results of cardiac autotransplantation as method to treat permanent atrial fibrillation and mitral disease. Eur J Cardiothorac Surg 2004;25:1025-31. [Crossref] [PubMed]
- Batista RJ, Santos JL, Takeshita N, et al. Partial left ventriculectomy to improve left ventricular function in end-stage heart disease. J Card Surg 1996;11:96-7; discussion 98. [Crossref] [PubMed]
- Sankar NM, Farnsworth AE. Left atrial reduction for chronic atrial fibrillation associated with mitral valve disease. Ann Thorac Surg 1998;66:254-6. [Crossref] [PubMed]
- García-Villareal OA, González R. Avoiding potential complications in left atrial reduction: partial heart autotransplantation. J Cardiovasc Surg (Torino) 2004;45:39-42. [PubMed]
- Stulak JM, Suri RM, Burkhart HM, et al. Surgical ablation for atrial fibrillation for two decades: are the results of new techniques equivalent to the Cox maze III procedure?. J Thorac Cardiovasc Surg 2014;147:1478-86. [Crossref] [PubMed]
- Kim HJ, Kim JB, Jung SH, et al. Surgical ablation of atrial fibrillation in patients with a giant left atrium undergoing mitral valve surgery. Heart 2016;102:1206-14. [Crossref] [PubMed]
- Byrd GD, Prasad SM, Ripplinger CM, et al. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation 2005;112:I7-13. [PubMed]


