
The ABCs of preventing hyperprogressive disease after immunotherapy: awareness, biomarkers, and combination
Hyperprogressive disease is a clinical term that describes the phenomenon of paradoxically accelerated tumor progression after initiation of treatment. Although there is no established definition for hyperprogressive disease, in clinical practice, we do experience patients who show unexpected rapid tumor expansion and poor prognosis even after a pulmonary resection (although it is rare). Primary tumors are believed to suppress the growth of micro-metastatic lesions in some patients, and the resection of primary tumors may accelerate metastatic growth in such patients (1). The incidence of hyperprogressive disease was rare in the treatment of non-small cell lung cancer (NSCLC) before the era of immunotherapy. However, following the recent introduction of immune-checkpoint inhibitors (ICIs) in routine clinical practice, this phenomenon is occasionally observed with ICI therapy and remains a shadow in this novel therapeutic approach in several types of malignancies (2), including NSCLC (3-5).
ICIs, including inhibitors against programmed cell death-1 (PD-1) or its associated ligand (PD-L1), exert anti-tumor effects through restoration of T cell activation. Based on the results of several randomized clinical trials, some PD-1/PD-L1 inhibitor monotherapies have been approved as therapeutic options for NSCLC patients in the second-line or later settings (6-11) and in the front-line setting if tumor cells have high expression of PD-L1 (tumor proportion score ≥50%) (12). PD-1/PD-L1 inhibitors combined with cytotoxic chemotherapies (13-16) or a CTLA-4 inhibitor—another type of ICI (17), have also shown promising efficacies in NSCLC patients in the front-line setting.
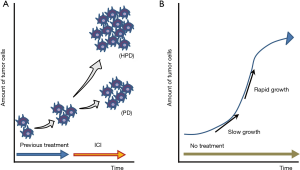
Since PD-1/PD-L1 inhibitors are becoming essential agents in the treatment of NSCLC, it is important to evaluate if these drugs are associated with hyperprogressive disease in a subset of NSCLC patients. If an association is identified, the identification of strategies to prevent or overcome hyperprogressive disease related to PD-1/PD-L1 inhibitors will be critical. However, distinguishing treatment-related hyperprogressive disease from natural progressive disease due to an aggressive disease phenotype, irrespective of treatment, can often be difficult. To identify patients with hyperprogressive disease during PD-1/PD-L1 inhibitor treatment, researchers have compared the tumor growth rate (TGR) during ICI treatment and the TGR during the pretreatment period in retrospective analyses (Figure 1A) (4). However, this evaluation may not accurately determine hyperprogressive disease. Even in the natural course of cancer, the speed of tumor growth may change depending on factors of the tumor cells themselves (as shown in a Gompertz growth curve, Figure 1B) and on factors related to the host (such as nutrition status of patients). Discontinuation of the previous treatment may also result in a “disease flare” (18), which complicates the accurate assessment of hyperprogressive disease. In addition, the incidence of hyperprogressive disease may also be underestimated for various reasons. For example, some patients may not be diagnosed with hyperprogressive disease if they progressed rapidly before the first scheduled computed tomography evaluation (4). Because of these issues, concluding whether hyperprogressive disease is more common in NSCLC patients treated with PD-1/PD-L1 inhibitors compared with other treatments was not possible.
To better address hyperprogressive disease during ICI treatment, Ferrara and colleagues performed a multicenter retrospective study involving eight French institutions (19). The researchers collected data from NSCLC patients who were treated with PD-1/PD-L1 inhibitor monotherapy (nivolumab, pembrolizumab, atezolizumab, or durvalumab) compared with a cohort of patients treated with single-agent chemotherapy (taxanes, pemetrexed, vinorelbine tartrate, or gemcitabine chlorohydrate) as second-line therapy. The TGR before and during treatment and the variation per month (ΔTGR) were calculated. Hyperprogressive disease was defined as disease progression at the first evaluation with ΔTGR exceeding 50%. Among 406 eligible patients treated with PD-1/PD-L1 inhibitor, 56 patients (13.8%) were classified as having hyperprogressive disease. In comparison, using the same definition of hyperprogressive disease, among 59 eligible patients treated with single-agent chemotherapy, only 3 (5.1%) were classified as having hyperprogressive disease. Importantly, patients who experienced hyperprogressive disease within the first 6 weeks of beginning PD-1/PD-L1 inhibitor treatment (n=23) had significantly lower overall survival (median 3.4 months) compared with patients who experienced progressive disease at the first evaluation (n=138, median overall survival 6.2 months; P=0.003).
Although the results of this study should be confirmed by independent research, these findings indicate that PD-1/PD-L1 inhibitor therapies will result in hyperprogressive disease in NSCLC patients at higher rates (approximately one out of seven patients in the study by Ferrara et al.) compared with traditional cytotoxic chemotherapies, and patients who develop hyperprogressive disease will have extremely poor survival. These findings lead to important questions for clinicians regarding how to prevent or mange hyperprogressive disease and how to improve treatment outcome with PD-1/PD-L1 inhibitors in treatment for NSCLC patients.
The first aspect in the strategy of managing hyperprogressive disease in NSCLC patients treated with PD-1/PD-L1 inhibitors is “Awareness”. Clinicians (and patients) should recognize that hyperprogressive disease may occur during the initial stage of PD-1/PD-L1 inhibitor therapies. In this context, it is important to not only calculate TGR after the initiation of PD-1/PD-L1 inhibitor therapy, but also compare it with TGR before PD-1/PD-L1 inhibitor therapy. Patients should be educated to declare any symptoms related to rapid tumor growth to their physicians, especially at the early phase of PD-1/PD-L1 inhibitor therapy. At the same time, however, clinicians should keep in mind that it is often difficult to distinguish hyperprogressive disease from pseudo-progression (progressive disease followed by complete or partial response/stable disease lasting for 6 months or longer). In the study by Ferrara et al., six pseudoprogressors were initially classified as having hyperprogressive disease on the first computed tomography scan (19).
The second aspect of successfully addressing hyperprogressive disease in NSCLC patients treated with PD-1/PD-L1 inhibitors involves “Biomarkers”. A translation research effort will need to be launched for the identification of effective biomarkers that may predict hyperprogressive disease prior to the initiation of PD-1/PD-L1 inhibitors. To date, several mechanisms that drive inherent resistance to PD-1/PD-L1 inhibitors have been reported. These mechanisms include tumor cell-mediated factors, such as the loss of tumor antigen expression, the absence of antigen presentation (through loss of HLA expression or alteration in the antigen processing machinery), or JAK1/2 aberrations. Other PD-1/PD-L1 inhibitor inherent resistance mechanisms involve immune cell-mediated factors, including expression of alternative immune checkpoints (such as LAG3 or TIM3), immunosuppressive cells (regulatory T cells, M2 macrophages, or myeloid-derived suppressor cells), or overexpression of immunosuppressive enzymes/cytokines/metabolites (e.g., IDO1/IL-10/adenosine, respectively) (2). The precise roles of these PD-1/PD-L1 inhibitor resistance mechanisms in the progression of hyperprogressive disease are unclear. However, several hypotheses have been suggested for the development of hyperprogressive disease during PD-1/PD-L1 inhibitor treatment. One review paper suggested that blockade of the PD-1/PD-L1 axis might functionally boost regulatory T cells, which also express PD-1, leading to an immunosuppressive tumor microenvironment (2). Notably, recent efforts have identified possible predictors for hyperprogressive disease such as tumor-infiltration by M2-like CD163+CD33+PD-L1+ clustered epithelioid macrophages (20) or a gene expression signature (21).
Finally, it is important to consider if there are any specific “Combination” regimens that are related to the prevention of hyperprogressive disease upon PD-1/PD-L1 inhibitor treatment. Although no prospective randomized trials have reported detailed evidence regarding hyperprogressive disease, it would be possible to suggest the occurrence of hyperprogressive disease in some trials if we carefully check the reported progression-free survival (PFS) curves as discussed by Ferrara et al. (19). As shown in Figure 2, the PFS curves from randomized trials comparing PD-1/PD-L1 inhibitors (monotherapy or in combination) versus cytotoxic drugs can be categorized into three groups. Interestingly, several randomized trials that compared immunotherapies with cytotoxic chemotherapies showed a crossover between the ICI arm and the chemotherapy arm at the early stage of the trials (Figure 2A). This crossing of PFS curves indicates that disease progression (and/or death) occurred at a higher rate in the ICI arm compared with the chemotherapy arm in the initial 3–6 months after the initiation of treatment, followed by an improvement in PFS compared with the chemotherapy arm.
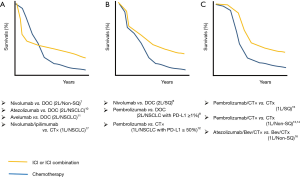
In 2008, the results of the IRESSA Pan-Asia Study showed similar crossing PFS curves in the comparison between front-line gefitinib [an epidermal growth factor receptor (EGFR) inhibitor] and carboplatin plus paclitaxel in East-Asian NSCLC patients with light- or never-smoking history (22). In the ITT (intention-to-treat) population, the PFS curves crossed at 6 months: the chemotherapy arm initially showed superior PFS and then the survival of the gefitinib arm was higher for the rest of the study period. This phenomenon was explained by the fact that NSCLC patients with EGFR mutation had better PFS in the gefitinib arm, while patients without EGFR mutation had better PFS in the chemotherapy arm; the mixture of these two populations resulted in crossing of the PFS curves.
In contrast to the EGFR story, in ICI trials, it is less likely that patients who benefit from immunotherapy and those who respond well to chemotherapy are mutually exclusive. One possible reason for the crossing of PFS curves in ICI randomized trials may be the higher incidence of hyperprogressors in patients who received PD-1/PD-L1 inhibitor treatment compared with patients receiving chemotherapy, as shown in the retrospective study by Ferrara et al. (13.8% vs. 5.1%). Then, what are the shared features of the randomized trials that showed the crossing of PFS curves? As summarized in Figure 2, PD-1/PD-L1 inhibitor combined with cytotoxic chemotherapy showed early separation of PFS curves with better outcome in the ICI arm (Figure 2C), while a front-line ICI combination therapy (nivolumab + ipilimumab) or later-line ICI monotherapies showed crossing of PFS curves (Figure 2A). Some ICI monotherapies showed the overlap of PFS curves in the early stage of trials, while ICI arms showed better outcomes in the later stage of trials (Figure 2B). Although Figure 2 may suggest that the crossing PFS curves seem to be common in nivolumab trials but not in pembrolizumab trials, the Keynote 045 study (second-line pembrolizumab monotherapy in urothelial carcinoma) also showed crossing PFS curves at 5 months (23). These PFS curves may suggest that PD-1/PD-L1 inhibitors should be used (in the front-line setting) with cytotoxic chemotherapy to avoid hyperprogressive disease (front-line “Combination” ICI therapy).
Due to the rapid progress in the development of immunotherapy for lung cancer, several treatment options are currently available or will be in development for these patients, including a choice of inhibitors (nivolumab, pembrolizumab, atezolizumab, durvalumab, or avelumab) and combination strategies. Because hyperprogressive disease is likely to be frequent in patients treated with PD-1/PD-L1 inhibitor therapies, clinicians have to consider the best treatment strategies using ICIs that are based not only on efficacy and avoiding adverse effects, but also on avoiding hyperprogressive disease.
Acknowledgements
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this letter.
Funding: This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (18K07336 to K Suda).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Demicheli R, Fornili M, Ambrogi F, et al. Recurrence dynamics for non-small-cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol 2012;7:723-30. [Crossref] [PubMed]
- Champiat S, Ferrara R, Massard C, et al. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol 2018;15:748-62. [Crossref] [PubMed]
- Kanai O, Fujita K, Okamura M, et al. Severe exacerbation or manifestation of primary disease related to nivolumab in non-small-cell lung cancer patients with poor performance status or brain metastases. Ann Oncol 2016;27:1354-6. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Kurman JS, Murgu SD. Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy. J Thorac Dis 2018;10:1124-8. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018;19:1468-79. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298-303. [Crossref] [PubMed]
- Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol 2018;4:1543-52. [Crossref] [PubMed]
- Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-Small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin Cancer Res 2019;25:989-99. [PubMed]
- Xiong D, Wang Y, Singavi AK, et al. Immunogenomic Landscape Contributes to Hyperprogressive Disease after Anti-PD-1 Immunotherapy for Cancer. iScience 2018;9:258-77.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]


