
Prevalence and clinical course of postoperative acute lung injury after esophagectomy for esophageal cancer
Introduction
Esophagectomy for esophageal cancer is associated with marked morbidity and mortality (1). Despite improvements in surgical technique and postoperative care, complications following esophagectomy, involving predominantly the cardiovascular system, occur in up to 50% of patients (2). Among pulmonary complications, acute lung injury (ALI) after esophagectomy, which is characterized by the acute onset of hypoxemia with radiographic pulmonary infiltrates without a clearly identifiable cause, has been reported (3,4). A few studies have reported that approximately 25–38% of patients develop ALI after esophagectomy for esophageal cancer (1,4,5); however, there are limited data on the clinical characteristics and outcomes of ALI following esophagectomy. The objective of this study was to investigate the prevalence and clinical course of ALI after esophagectomy.
Methods
Data were collected from all consecutive patients diagnosed with ALI after esophagectomy for esophageal cancer at Samsung Medical Center (a 1,989-bed, university-affiliated, tertiary referral hospital in Seoul, South Korea) from January 2012 through March 2017 and retrospectively analyzed. The institutional review board of the Samsung Medical Center approved the review and publication of information obtained from the patients’ records (SMC 2017-04-068). Informed consent was waived because of the observational nature of the study.
Diagnosis of ALI after esophagectomy
During the study period, ALI after esophagectomy was diagnosed by (I) sudden onset of respiratory distress within 7 days after surgery; (II) diffuse pulmonary infiltrates on chest computed tomography (CT); (III) impaired oxygenation with partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio (PF ratio) <300 mmHg; (IV) symptoms not fully explained by cardiac failure or fluid overload. Other causes of respiratory distress such as respiratory or systemic infection were excluded. Severity of ALI was classified by the Berlin definition for acute respiratory distress syndrome (ARDS) in patients receiving mechanical ventilation (MV) (6).
Data collection
The medical records of the patients were reviewed and clinical data were extracted. Preoperative data included demographic characteristics, body mass index, smoking history, American Society of Anesthesiologists physical status, comorbidities (chronic obstructive pulmonary disease, hypertension, diabetes mellitus, chronic liver disease, and other malignancies), pulmonary function tests, location of tumor, histologic type, clinical stage, and any neoadjuvant treatment (chemotherapy, radiotherapy, or concurrent chemoradiotherapy). Intraoperative data included approach type, lymph node (LN) dissection, total operation time, one-lung ventilation (OLV) time, peak airway pressure during OLV, tidal volume (Vt) during OLV, intraoperative volume, transfusion, and bleeding volume.
The following variables were measured when ALI diagnosed: Sequential Organ Failure Assessment (SOFA) score (7), PF ratio, lung injury score (LIS), serum C-reactive protein (CRP), and arterial blood gas analysis. We also extracted the following data about treatment modalities after ALI development: MV settings (FiO2, positive end-expiratory pressure, and support pressure), monitored Vt, antibiotics, vasopressor, tracheostomy, continuous renal replacement therapy (CRRT), and extracorporeal membrane oxygenation (ECMO). Regarding disease course and treatment outcomes, we extracted data on intensive care unit (ICU) admission rate, complications (arrhythmia, delirium, superimposed infection, and surgical site complications), weaning from MV, MV days, length of stay in ICU and hospital, and 28-day, ICU and hospital mortality.
Data are presented as the median and interquartile range (IQR) for continuous variables and as frequency (percentage) for categorical variables. Data were analyzed using IBM SPSS Statistics for Windows, version 23.0 (Armonk, NY, USA).
Results
During the study period, a total of 1,132 patients underwent esophagectomy for esophageal cancer and 52 (4.6%) patients developed ALI within 1 week after surgery. Of the 52 patients, all were male, most of them had smoking history (94%), and the median age was 65 years (IQR, 58–71 years). The most common location of the tumor was middle third (46%) and the most common histologic type was squamous cell carcinoma (98%). Sixteen (31%) patients received neoadjuvant treatment before the operation (Table 1). Intraoperative data are summarized in Table 2. Regarding approach type, 43 (83%) patients underwent transthoracic esophagectomy and 9 (17%) underwent minimal invasive esophagectomy. Two-field LN dissections (77%) were performed more frequently than three-field LN dissections (23%).
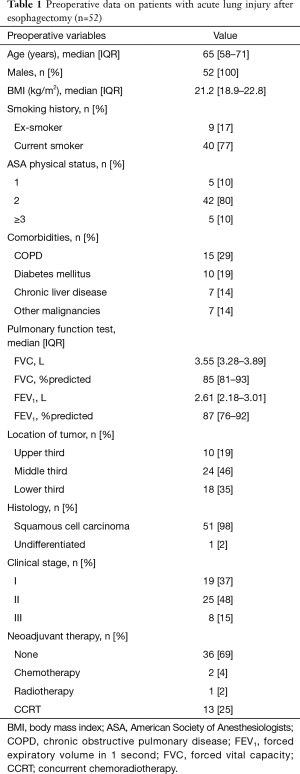
Full table
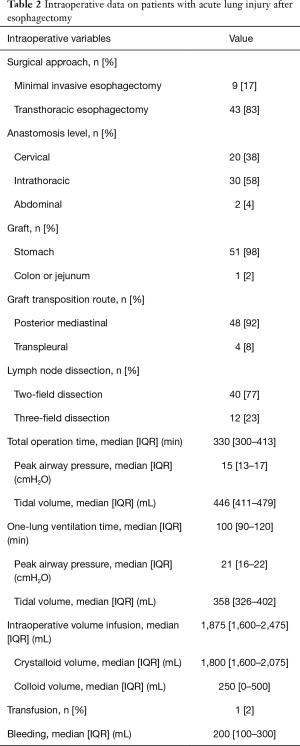
Full table
At the time of ALI diagnosis, the median LIS and PF ratio of all patients were 1.8 (IQR, 1.0–2.0) and 255 (IQR, 190–310), respectively. Seventeen (33%) patients required MV support; 7 (13%) were classified as moderate ARDS and none were classified as severe ARDS by the Berlin definition. On laboratory data, the median CRP level of patients was 14.0 mg/dL (IQR, 12.1–19.5 mg/dL) (Table 3).
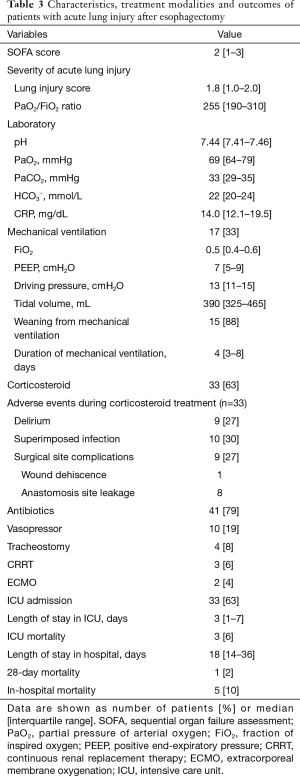
Full table
Over the study period, 17 (33%) patients required MV support. Of the 17 patients receiving MV support, 15 (88%) were successfully weaned from MV after median of 4 days (IQR, 3–8 days). Of the remaining two patients who could not be weaned from MV, one died from respiratory failure despite ECMO treatment and the other died from hypovolemic shock due to gastrointestinal bleeding. All 35 patients that did not receive MV support survived. Corticosteroids were used in 33 (63%) patients and empirical antibiotics were administered to 41 (79%) patients. In addition, ECMO and CRRT treatment were used at the ICU in 3 (6%) and 2 (4%) patients respectively. During corticosteroid treatment, superimposed infections were observed in 10 (30%) patients and surgical site complications were observed in 9 (27%), which included wound dehiscence (n=1) and anastomosis site leakage (n=8). The overall in-hospital mortality rate was 10% (Table 3).
Discussion
In this observational study, we investigated the clinical course of patients with ALI after esophagectomy for esophageal cancer. The overall prevalence of ALI was 4.6%. About one-third of the study population required MV support; these patients had mild to moderate ARDS and most of them were weaned from MV successfully. Overall in-hospital mortality was 10%.
Because of the clinical significance of ALI after esophagectomy, several studies have been conducted to evaluate its pathophysiology. Release of pulmonary cytokines and inflammatory mediators (8,9) and ventilator-induced lung injury during OLV (10,11) have been suggested as possible mechanisms. In this study, CRP levels were elevated when ALI was diagnosed, consistent with previous studies reporting that systemic and pulmonary inflammation is associated with ALI after esophagectomy (9,12,13). In addition to inflammation, OLV during esophagectomy is suggested to be a significant factor underlying the development of ALI (14). Large tidal volume and high airway pressure during OLV is associated with an increased risk of postoperative ALI (15,16). In the present study, however, the overall prevalence of ALI was 4.6%, which is relatively low compared with previously reported rates of 25–38% (1,4,5). We ascribe our lower prevalence rate in part to our lung protective ventilation strategy during OLV, which consisted of FiO2 0.5, Vt 6 mL/kg, positive end-expiratory pressure 5 cmH2O, and pressure-controlled ventilation (17). This is supported by a recent meta-analysis showing that the use of low tidal volume resulted in a lower incidence of ARDS in patients undergoing OLV (18).
Despite a number of studies, no drugs that directly target the underlying pathophysiological mechanisms implicated in the development of ARDS have been identified (19); however, the role of corticosteroid treatment in the management of ARDS has been systematically studied. The beneficial effects of corticosteroids in ARDS are consistent with the hypothesis that fibroproliferation is an early response to lung injury that is inhibited by early low-dose corticosteroid treatment (20). However, there is limited information on the optimal therapy for ALI after esophagectomy. Despite the beneficial effects of early low-dose corticosteroid treatment in ARDS, the suppressant effect of corticosteroids on wound healing and the immune response raises concern in patients undergoing surgery. One meta-analysis analyzing the effect of perioperative glucocorticoid administration on postoperative complications following esophagectomy showed that the rate of severe infection between the control and methylprednisolone-treated groups were similar (21). In the present study, however, about two-thirds of the patients received corticosteroid treatment when ALI was diagnosed; among these patients, 9 (27%) developed surgical site complications and 10 (30%) had superimposed infections. Therefore, the use of corticosteroids in patients with ALI following esophagectomy requires attention to the occurrence of surgical site complications and close surveillance to identify new infections and treat them promptly.
There are several potential limitations to our study that should be acknowledged. A major limitation is the fact that we did not systematically screen patients with acute hypoxemic respiratory failure within 7 days after esophagectomy. More severely ill patients might not have undergone chest CT scanning even if they were strongly suspected to have ALI. In addition, given the retrospective nature of our study, there is the inherent possibility that selection bias may have influenced the significance of our findings. Furthermore, our study population was from a single institution with the largest number of esophageal cancer surgeries performed in Korea for the last 5 years, which limits the generalization of our findings to other institutions.
In summary, the prevalence and mortality of ALI following esophagectomy in our study were relatively low compared with previous reports. These might be associated with lung protective ventilation strategy during surgery and corticosteroid treatment. However, the use of corticosteroids in patients with ALI following esophagectomy requires attention to the occurrence of surgical site complications and close surveillance to identify new infections. Further evaluation with a prospective study with a large sample size is needed to confirm our observations.
Acknowledgements
Funding: This work was supported by a Samsung Medical Center grant (OTA1602901).
Footnote
Conflicts of Interest: The authors no conflict of interest to declare.
Ethical Statement: The institutional review board of the Samsung Medical Center approved the review and publication of information obtained from the patients’ records (SMC 2017-04-068). Informed consent was waived because of the observational nature of the study.
References
- Boshier PR, Marczin N, Hanna GB. Pathophysiology of acute lung injury following esophagectomy. Dis Esophagus 2015;28:797-804. [Crossref] [PubMed]
- Morita M, Yoshida R, Ikeda K, et al. Advances in esophageal cancer surgery in Japan: an analysis of 1000 consecutive patients treated at a single institute. Surgery 2008;143:499-508. [Crossref] [PubMed]
- Park SY, Lee HS, Jang HJ, et al. Efficacy of intraoperative, single-bolus corticosteroid administration to prevent postoperative acute respiratory failure after oesophageal cancer surgery. Interact Cardiovasc Thorac Surg 2012;15:639-43. [Crossref] [PubMed]
- Tandon S, Batchelor A, Bullock R, et al. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth 2001;86:633-8. [Crossref] [PubMed]
- Howells P, Thickett D, Knox C, et al. The impact of the acute respiratory distress syndrome on outcome after oesophagectomy. Br J Anaesth 2016;117:375-81. [Crossref] [PubMed]
- Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Katsuta T, Saito T, Shigemitsu Y, et al. Relation between tumour necrosis factor alpha and interleukin 1beta producing capacity of peripheral monocytes and pulmonary complications following oesophagectomy. Br J Surg 1998;85:548-53. [Crossref] [PubMed]
- Sakamoto K, Arakawa H, Mita S, et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine 1994;6:181-6. [Crossref] [PubMed]
- Jordan S, Mitchell JA, Quinlan GJ, et al. The pathogenesis of lung injury following pulmonary resection. Eur Respir J 2000;15:790-9. [Crossref] [PubMed]
- Tugrul M, Camci E, Karadeniz H, et al. Comparison of volume controlled with pressure controlled ventilation during one-lung anaesthesia. Br J Anaesth 1997;79:306-10. [Crossref] [PubMed]
- Morita M, Yoshida R, Ikeda K, et al. Acute lung injury following an esophagectomy for esophageal cancer, with special reference to the clinical factors and cytokine levels of peripheral blood and pleural drainage fluid. Dis Esophagus 2008;21:30-6. [Crossref] [PubMed]
- Yamada T, Hisanaga M, Nakajima Y, et al. Serum interleukin-6, interleukin-8, hepatocyte growth factor, and nitric oxide changes during thoracic surgery. World J Surg 1998;22:783-90. [Crossref] [PubMed]
- Baudouin SV. Lung injury after thoracotomy. Br J Anaesth 2003;91:132-42. [Crossref] [PubMed]
- Fernandez-Perez ER, Keegan MT, Brown DR, et al. Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomy. Anesthesiology 2006;105:14-8. [Crossref] [PubMed]
- Jeon K, Yoon JW, Suh GY, et al. Risk factors for post-pneumonectomy acute lung injury/acute respiratory distress syndrome in primary lung cancer patients. Anaesth Intensive Care 2009;37:14-9. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- El Tahan MR, Pasin L, Marczin N, et al. Impact of Low Tidal Volumes During One-Lung Ventilation. A Meta-Analysis of Randomized Controlled Trials. J Cardiothorac Vasc Anesth 2017;31:1767-73. [Crossref] [PubMed]
- Jeon K. Pharmacotherapy for Acute Respiratory Distress Syndrome: Limited Success to Date. Tuberc Respir Dis (Seoul) 2017;80:311-2. [Crossref] [PubMed]
- Meduri GU, Muthiah MP, Carratu P, et al. Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation 2005;12:321-38. [Crossref] [PubMed]
- Gao Q, Mok HP, Wang WP, et al. Effect of perioperative glucocorticoid administration on postoperative complications following esophagectomy: A meta-analysis. Oncol Lett 2014;7:349-56. [Crossref] [PubMed]


