Heart-lung transplantation: adult indications and outcomes
Adult indications
The international society of heart and lung transplantation (ISHLT) registry data shows idiopathic pulmonary arterial hypertension (IPAH) was the most common indication followed by congenital heart disease (CHD) and cystic fibrosis (CF) in 1982-1991 (n=959) (1). The data from the most recent period from 2002 to June of 2012 (n=829), however, shows that CHD became the most common indication followed by IPAH. The proportion decreased for CF and increased for acquired heart disease.
Decrease of the proportion for IPAH is in agreement with our recommendation. We have shown an excellent outcome with double lung transplant and combined heart-lung transplantation for patients with IPAH when indication for HLTx is inotropic dependency for right ventricular support and/or concomitant left ventricular dysfunction (2). However, further study is necessary to determine whether double lung transplant is sufficient even for patients with inotropic dependency for right ventricular failure.
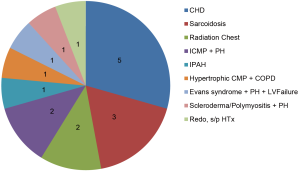
We have experienced 17 heart-lung transplants from 2005 to 2012 for the indications shown in the Figure 1.
In our experience, CHD was the most common indication for HLTx, including transposition of the great arteries s/p Mustard operation with systemic ventricular failure and pulmonary hypertension, Shone’s syndrome with pulmonary hypertension, Eisenmenger’s syndrome s/p ventricular and/or atrial septal defect repair, polysplenia syndrome, etc. Because of previous multiple surgeries and potential sensitization due to multiple blood transfusion and use of homograft, complex CHD is a challenging indication and only experienced surgeons who are familiar with CHD should perform the surgery.
Case presentation 1
A 27-year-old man with Shone’s syndrome had repair of coarctation of the aorta and mitral valve replacement three times previously. At the time of HLTx, he had been on mechanical ventilation with FiO2 70% and inhaled nitric oxide at 40 ppm for 5 days and he was on 10 mcg/kg/min of dopamine and 0.1 mcg/kg/min of norepinephrine to maintain his blood pressure. He had kidney failure on continuous veno-venous hemodialysis. Despite his critically ill condition, he continued to be listed and underwent a combined heart-lung transplantation utilizing organs from a 40-year-old female donor. Total ischemic time was 274 minutes, warm ischemic time (organ in the chest till reperfusion) 42 minutes, and cardiopulmonary bypass time 336 minutes. Postoperatively, due to preoperative low output, he required bilateral below-knee amputation due to ischemic legs. His hospital stay was 151 days. However, the patient is now doing well more than 4 years postoperatively with normal heart, and lung functions. We believe this case suggests the limit for a successful outcome after HLTx.
We have experienced three sarcoidosis. Pulmonary and cardiac involvements were common. As in other diseases, careful assessment of other organ function is important. In fact, we have performed a combined heart, lung and liver transplantation for a patient who had congestive liver cirrhosis in addition to end-stage heart and lung diseases as shown below.
Case presentation 2
A 49-year-old man had pulmonary and cardiac sarcoidosis with severe pulmonary hypertension and liver cirrhosis on Mirinone 0.375 mcg/kg/min. A combined heart-lung-liver transplantation was performed utilizing organs from an 18-year-old male donor. The total ischemic time was 164 minutes and cardiopulmonary bypass time was 264 minutes. The patient is doing well with normal heart, lung and liver functions more than 5 years postoperatively. Thus, sarcoidosis seems a good indication for HLTx and perhaps liver or kidney transplant concomitant with HLTx.
As a unique group of patients, we have experienced two patients who had extensive, mantle radiation to the chest for Hodgkin lymphoma. Both patients had pleurodesis and had extensive, severe adhesions. In our limited experience, radiation chest may have to be considered as a relative contraindication for HLTx.
As the ISHLT registry data indicated, acquired heart disease is increasing as an indication for HLTx. We had two ischemic cardiomyopathy and pulmonary hypertension and one hypertrophic cardiomyopathy and COPD. These three patients were on left or bi-ventricular assist devices (VAD) preoperatively. As more and more patients with end-stage heart failure are managed with VADs as a bridge-to-heart transplantation, we need to carefully monitor their lung status including pulmonary hypertension. Especially when a patient is on RVAD, chronic thromboembolic pulmonary hypertension can occur due to thrombus formed in the RVAD while waiting for a heart transplant. In such situation, the patient may have to be placed in heart-lung transplant list rather than heart transplant only. Thus, pulmonary hypertension and/or end-stage lung disease in the setting of predominant left and/or biventricular failure can be a good indication for HLTx.
Case presentation 3
A 47-year-old man who had bi-ventricular assist device for his hypertrophic cardiomyopathy. He was found to have severe pulmonary hypertension and Child B, MELD 12, liver cirrhosis. He successfully underwent a HLTx from an 39-year-old male donor. Total ischemic time was 192 minutes, warm ischemic time (organ in the chest till reperfusion) 36 minutes, and cardiopulmonary bypass time 185 minutes.
On the other hand, only 1 patient had IPAH (6%) as an indication for HLTx in our experience. During the same time period, we have performed 5 double lung transplants for IPAH. In patients with pulmonary hypertension and right ventricular failure, we need to determine if the right ventricle is sick enough to warrant HLTx. Based on our experience, both acute and chronic right ventricular failure due to severe pulmonary hypertension and hypoxia, which requires inotropic support and/or even veno-arterial extracorporeal membrane oxygenation, can be reversed after double lung transplantation by normalizing the pulmonary vascular resistance. Therefore, virtually all patients with IPAH should receive double lung transplantation, and HLTx is not necessary as far as the left ventricle is normal. Left ventricular dysfunction in the setting of pulmonary hypertension and right ventricular failure, can be the reason for HLTx. The question is what degree of left ventricular dysfunction necessitates HLTx. In our experience, LVEF 30-35% is still sufficient for double lung transplantation alone if the right heart catheterization shows good cardiac index (e.g., >2.2 L/min/m2) and low filling pressures (e.g., PCWP and/or LVEDP ≤15 mmHg).
Patients who have end-stage lung disease with repairable cardiac diseases can be treated by lung transplantation and concomitant cardiac surgery such as coronary artery bypass surgery, valve repair/replacement, repair of CHDs, etc.
Regarding recipient age limit, in our opinion, there should not be a rigid chronological age limit. The ISHLT registry data also suggests the age limit is increasing. Almost 5% of HLTx recipient were age 60 and older and some were 65 and older from 2006 to 2012 (1). If a patient has good other organ functions and the physiological age seems reasonable, we would consider up to 70 years old or so for HLTx. Regarding donor age limit, we would consider up to around 60 years old in agreement with the ISHLT registry data.
Adult outcomes
The ISHLT registry data shows survival rates of 71% at 3 months, 63% at 1 year, 44% at 5 years and 31% at 10 years. Recipients who survived the first year had a median survival of 10.0 years. A multivariable analysis of risk factors for 1-year mortality showed IPAH as favorable diagnosis [hazard risk (HR): 0.78, 95% confidence interval (CI): 0.63-0.96, P=0.0171] and donor age as a significant independent predictor for 1-year mortality, although it did not demonstrate recipient age as a predictor.
Variables influencing survival in heart-lung recipients are not well established. We have analyzed 542 adult patients who received heart-lung transplantation from 1995 to 2011 in the UNOS database (3). Although the use of ECMO as a bridge to lung transplantation is an accepted therapy for patients with end-stage lung disease (4), the role of ECMO as a bridge to combined adult HLT has only been described in case reports (5,6). Our multivariate analysis demonstrated that preoperative use of extracorporeal membrane oxygenation (ECMO, HR: 3.820, 95% CI: 1.600 to 9.112, P=0.003) and mechanical ventilator (HR: 2.011, 95% CI: 1.069 to 3.784, P=0.030) is a risk factor for mortality and recipient female gender (HR: 0.754, 95% CI: 0.570 to 0.998, P=0.048) is associated with better survival (3).
Mechanical circulatory support bridge-to-HLTx
Of the 17 patients in our experience, 5 patients were supported with mechanical circulatory support at the time of HLTx. We have successfully performed HLTx for 2 patients who were on veno-arterial ECMO preoperatively. Two other patients were on bi-ventricular assist deivces and one on left ventricular assist device. All patients survived HLTx surgery with 100% survival at 30, 90, 180 and 300 days with 1-year survival rate of 80%. Good outcomes can be achieved even for patients who required mechanical circulatory support including ECMO preoperatively.
Case presentation 4
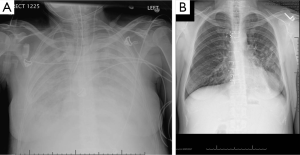
A 41-year-old female who has chronic lung infections due to Evans syndrome and hypogammaglobulinemia developed severe pulmonary hypertension, right ventricular failure and hypoxia. She was listed for double lung transplantation, however, she was admitted with worsening shortness of breath and lower extremity edema. She was intubated and mechanically ventilated with FiO2 100%, PEEP 15 cm H2O and inhaled nitric oxide, and required inotopes for her right ventricular failure (Figure 2A). She developed cardiac arrest in the intensive care unit and was placed on veno-arterial ECMO. Her echo showed LVEF 10%. She was then listed for heart-lung transplant. After ECMO support for 8 days, heart-lung transplantation was performed using the organs from a 27-year-old male donor. The ischemic time was 136 minutes, the warm ischemic time 43 minutes and cardiopulmonary bypass time 215 minutes. Her ECMO was weaned in the OR. She was extubated on postoperative day #1 (Figure 2B). The ICU stay was 5 days. She was discharged home on postoperative day #32. Her right heart catheterization showed RA 2, PAP 32/9 (21), PCWP 10, and cardiac index 4.35 L/min/m2. Her PFT showed FVC 2.89 L (78%) and FEV1 2.21 L (74%). She is doing well as of 1 year postoperatively. This case suggests that preoperative ECMO should not be an absolute contraindication, and with appropriate expertise, a good outcome can be achieved.
As mentioned above, we have experienced three sarcoidosis patients who had both left ventricular dysfunction and end-stage lung disease with severe pulmonary hypertension. A patient even had liver involvement with liver failure requiring a combined heart-lung and liver transplantation. All three patients are surviving 2-5 years (100% survival rate at 1 and 5 years). Sarcoidosis is a good indication for HLTx if patients have both heart and lung failure, but we need to assess other organ dysfunctions for additional organ transplants. On the other hand, both patients who had prior mantle radiation to the chest did not survive for 1 year (0% 1-year survival).
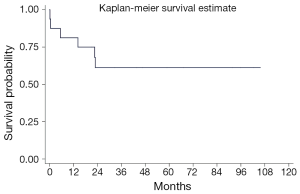
Overall, 3-month, 1-year and 5-year survival rates were 88%, 81% and 61%, respectively (Figure 3). These results are favorable when compared to the ISHLT registry data which showed 71% at 3 months, 63% at 1 year and 44% at 5 years although our patient cohort seemed to be higher-risk patients because No. 1: only 1 patient had IPAH (best indication for better outcomes), No. 2: 5 patients (29%) were on mechanical circulatory support including 2 ECMOs and 3 VADs at the time of HLTx, No.3: 2 patients had radiation chest. No. 4: 4 patients were on mechanical ventilation and in profound cardiogenic shock.
To achieve the best possible outcomes, surgeons need to do the best possible job in the OR. First, surgeons need to achieve good hemostasis after explantation of the heart-lung block from the recipient chest before starting implantation. Second, tracheal anastomosis should be done by making the donor trachea as short as possible. The surrounding tissue of the recipient and donor trachea should be preserved as much as possible and it should be used to cover the tracheal anastomosis. Third, the aortic anastomosis should be done immediately after the tracheal anastomosis, and the heart should be reperfused immediately after the aortic anastomosis to minimize the total and warm ischemic time. Fourth, preservation/protection and management of the heart and lung are important.
In summary, as the last resort for patients with end-stage heart and lung failure, combined heart-lung transplantation remains an excellent, viable therapy, and excellent outcomes can be achieved when the patient selection is appropriate for surgical expertise.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [PubMed]
- Toyoda Y, Thacker J, Santos R, et al. Long-term outcome of lung and heart-lung transplantation for idiopathic pulmonary arterial hypertension. Ann Thorac Surg 2008;86:1116-22. [PubMed]
- Jayarajan SN, Taghavi S, Komaroff E, et al. Impact of extracorporeal membrane oxygenation or mechanical ventilation as bridge to combined heart-lung transplantation on short-term and long-term survival. Transplantation 2014;97:111-5. [PubMed]
- Toyoda Y, Bhama JK, Shigemura N, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg 2013;145:1065-70; discussion 1070-1. [PubMed]
- Gregoric ID, Chandra D, Myers TJ, et al. Extracorporeal membrane oxygenation as a bridge to emergency heart-lung transplantation in a patient with idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2008;27:466-8. [PubMed]
- Strueber M, Hoeper MM, Fischer S, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant 2009;9:853-7. [PubMed]