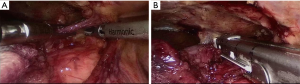
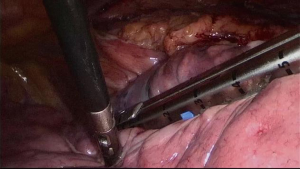
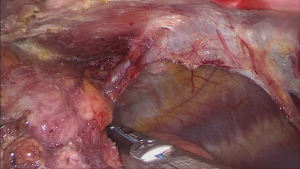
Thoracoscopic thymectomy with partial superior vena cava resection for locally advanced thymomas
Introduction
Video-assisted thoracoscopic surgery (VATS) has been increasingly used for the treatment of thymic malignancies (1). Recent studies suggest that VATS thymectomy for early stage thymic tumors may have comparable oncological results as open surgery, in addition to better perioperative outcomes (2-9). It is thus considered an acceptable alternative approach for early stage thymic tumors (10). However, few studies have focused on the safety and efficacy of VATS for locally invasive thymic tumors.
Oncological outcomes aside, thymic tumors with limited invasion into neighboring structures such as pericardium, lung, or phrenic nerve are readily resectable under VATS. But it is technically challenging to achieve complete tumor resection when the lesion has extensive local invasion, especially the great vessels such as the innominate veins. When the superior vena cava (SVC) is invaded, bypass surgery with prosthetic vessels is often required to remove the tumor, although in some circumstances lateral wall tangential resection is also a potential alternative when vessel invasion is limited and R0 resection can be achieved (11-14). It was recently reported that partial SVC resection for lung cancer might be feasible under VATS (15). However, this has never been accomplished in thoracoscopic surgery for mediastinal tumors. We hereby report our initial experience in VATS thymectomy together with partial the SVC for locally advanced thymic malignancies.
Methods
Patients and parameters
From August 2017 to October 2018, 4 patients who underwent VATS thymectomy with partial SVC resection by a single group of surgeons at the Shanghai Chest Hospital were retrospectively retrieved from a prospectively recorded database. Because only de-identified data were used for the study, informed consent was waived by IRB. Clinical data included patient gender, age, co-morbidity, tumor size, histologic type according to the WHO classification (16), TNM stage according to the International Association for the Study of Lung Cancer/International Thymic Malignancy Interest Group (IASLC/ITMIG) TNM staging proposal (17), surgical approach, co-resection, operative time, amount of blood loss, duration of chest tube drainage, length of post-operative hospital stay, morbidity and mortality. Follow-up results were also examined for disease recurrence.
Surgical techniques
Bilateral approach
Under general anesthesia and double-lumen intubation, patient was firstly prepared for the left-sided operation. Patient’s torso was brought to the left side of the operating table. Mats were placed under the scapula to the iliac crest, making the left hemithorax 45-degree elevated. Patient’s left arm was tucked under his torso. A camera port was placed in the 5th intercostal space in the mid axillary line. A right-hand port was made in the third intercostal space at the anterior axillary line, aiming toward the tumor bed, and a left-hand port was made in the 4th intercostal space at the midclavicular line. Artificial pneumothorax with carbon dioxide insufflation at a flow of 10 mL/min and a set pressure of 6 to 8 mm Hg was applied for enlarging the surgical field. Exploration was performed first to identify the lesion and to inspect the thoracic pleural cavity for occult pleural dissemination. Dissection started by opening the retrosternal space to the contralateral mediastinal pleura, followed by dissecting the mediastinal fatty tissue upward from the left cardiophrenic angle along the phrenic nerve. The inferior border of the left innominate vein was identified once the thymus was dissected from the aortopulmonary window. Continuing dissection of the left upper pole of the thymus exposed the superior border of the left innominate vein. An endo-stapler was then used to divide the vein when tumor invasion was suspected.
The patient was then prepared for the right-sided thoracoscopy. A subxyphoid port was added besides the usual three ports. Usually, endo-staplers were used to perform wedge resection of the lung if invasion was suspected. Disconnecting the internal-mammary vein from the right innominate vein helps provide a better view to dissect the right upper pole of the thymus. Pericardium was usually opened to expose the whole length of the SVC. After careful dissection of surrounding tissue, the SVC was almost isolated and the invaded lateral wall, together with the cordial part of the left innominate vein, could be cut off by an endo-stapler. Then the whole sample was retrieved via the subxyphoid port through a retrieving bag (Figures 1-3).
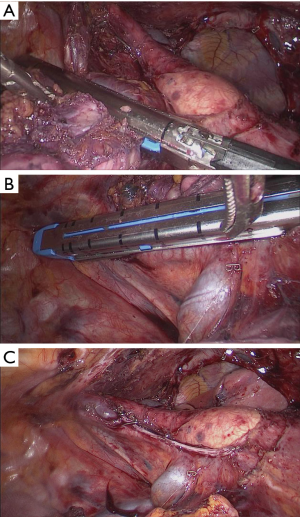
Right-sided approach
The process was similar to what has been described above except for two parts. First, ligation of the left innominate vein was performed via the subxyphoid port if invasion was suspected. Second, if tumor invasion into the contralateral lung was found intraoperatively, wedge resection through the subxyphoid port was still possible (Figures 4 and 5).
Statistical analysis
Descriptive statistics were used to summarize pertinent study information. Continuous variables are presented as median (range) and categorical variables as frequency with relative percentage.
Results
Patient demographics and clinical features
Four patients with partial SVC resection via VATS were identified. Patient demographics and clinical features are illustrated in Table 1. Two of them were male and the other two were female. The average age was 54 years old. One patient had concomitant Osserman grade IIB myasthenia gravis, one with aplastic anemia, and another one with pemphigoid. All of them had pathologically confirmed IASLC/ITMIG stage III thymic tumors invading the SVC as well as other neighboring structures. There was one squamous cell carcinoma and three type B thymomas.

Full table
Peri-operative outcomes and short-term follow-up
Due to the location and extension of their tumors, two patients underwent surgery via bilateral approach, and two through right-sided approach only. Median operation time was 220.5 [160–310] minutes, with median amount of blood loss as 50 [50–200] mL. Median duration of chest tube drainage and post-operative hospital stay were 4.5 [2–7] days and 6 [5–12] days, respectively. Noninvasive ventilation support was used for the patient with myasthenia gravis on the first postoperative day. No major complication was encountered after surgery, and the patients were discharged uneventfully within 7 days except for the one with pemphigoid who had prolonged hospital stay due to wound infection. Adjuvant radiation was applied in all 4 patients according to our existing protocol. And no recurrence was detected with the longest follow-up time of 14 months.
Discussion
Median sternotomy has long been the standard approach for surgical resection of thymic malignancies. But thoracoscopic surgery is increasingly used for the treatment of early-stage thymic tumors in recent years (2,3,9,18,19). A variety of studies suggested that it is associated with comparable R0 resection rate and non-inferior survival, in addition to less post-operative complications, less blood loss, shorter length of hospital stay compared to open surgery (2-9). Nevertheless, the use of VATS is mostly limited to early stage lesions (Masaoka stage I-II or IASLC/ITMIG stage I) (20). For locally invasive tumors, limited clinical data is available yet to assess the safety and efficacy of VATS. Median sternotomy is still the standard approach for tumors invading mediastinal great vessels, especially those into the SVC, either for prosthetic vessel replacement or for tangential lateral wall resection. To our knowledge, this has never been accomplished via minimally invasive approach. We believe the current series is the first to demonstrate the safety and feasibility of VATS partial SVC resection for locally advanced thymic tumors with major blood vessel invasion.
With the advent of minimally invasive surgical techniques, extended resection is sometimes feasible in experienced hands. Burt and colleagues (18) analyzed determinants of R0 resection among patients undergoing minimally invasive or open thymectomy using the ITMIG retrospective global database. Their results showed that resectability was not associated with surgical approach in well matched cases, including 33 Masaoka stage III tumors. Previously we also reported a case of a recurrent thymoma invading left innominate vein and phrenic nerve which was successfully resected under VATS (21). Xu et al. (15) reported SVC partial resection via VATS in a lung cancer patient in 2016. However, thymic tumors invading into the SVC is an even more challenging scenario. As was shown in this series, SVC invasion by thymic tumors is often associated with other major structure involvement, such as the left and/or right innominate veins, pericardium, phrenic nerve, and the neighboring lung.
According to the IASLC/ITMIG TNM staging (17), SVC invasion is classified into T3 diseases, together with lung, phrenic nerve, extrapericardial pulmonary vessels, innominate vein, and chest wall. In the current series of SVC plasty, all 4 patients received multiple structures co-resection under VATS. These included most of those listed in the T3 category, from limited wedge resection of the lung to phrenic nerve, and major vascular structures such as innominate veins. Even in open surgery, complete tumor removal together with such extensive resection is technically demanding (11-14). Post-operative complications are not uncommon, leading to certain risks of mortality after surgery. Morbidity rate was reported to be as high as 63%, and mortality from 0% to 5.5%, although not all of them contain detailed results specifically for thymic tumors (Table 2). In contrast, there was no mortality or major morbidity after VATS resection in the current series. Although this does not necessarily mean that minimally invasive surgery is better, presuming that lesions requiring open surgery might be more extensive, it does show that MIT is a promising surgical approach in selected patients. Besides, adjuvant therapies are often needed to reduce the risk of disease recurrence in such patients (22). In this concern, minimally invasive surgery is extremely desirable to reduce surgical trauma, enhancing patients’ recovery, and facilitating post-operative treatment.

Full table
Successful accomplishment of extensive VATS resection for locally advanced thymic tumors depends on careful evaluation of the patient’s condition and appropriate selection of surgical approach. Bilateral VATS approach was chosen for two of the patients, one with modified Osserman grade IIB MG and the other with a large tumor 6.7 cm in diameter abutting mediastinal pleura on both sides. First according to preoperative evaluation, complete resection via VATS seemed possible based on our previous experience and was thus worth trying. Conversion to open surgery was prepared whenever oncologic principles might be compromised or uncontrollable bleeding happened. Then, the patient with MG could benefit from minimal invasive procedure by minimizing the risk of postoperative myasthenia crisis. Lastly, had the tumor been staged as unresectable before operation, induction chemoradiation would be the choice according to our treatment protocol at the Shanghai Chest Hospital. Although all the tumors turned out to have extensive invasion in this series, lateral wall involvement of the SVC was very limited (less than 3 cm in length and less than 1/4 of the circumference). Otherwise conversion to open sternotomy and resection of the SVC with prosthetic vessel reconstruction would be needed to ensure complete resection of the tumor.
A left-sided VATS was performed first for these two patients for three reasons. First, the main part of the tumor was located right to the midsternal line with invasion mostly into the right thoracic cavity. We tended to start with the easier part of the procedure and by then it became safer and more convenient to manage with a larger operating space. Second, the left-sided VATS offers a better view of the left innominate vein. Without the obstruction of the tumor mass, inspection and dissection of this vessel could be readily accomplished under VATS, according to our previous experience (21). After division of the left innominate vein and dissection of left lobe of the thymus, the tumor could be mobilized to the right side, making further dissection feasible. Third, as the right phrenic nerve was most likely to have been invaded by the tumor in these two cases, it was critically important to preserve the left phrenic nerve so as to avoid the disastrous consequence of bilateral diaphragm paralysis.
The most challenging part of the right side procedure was the partial resection of the SVC. Before surgery, careful study of the critical structures on CT scan and MRI is mandatory to evaluate the resectability of the tumor. Since most thymic tumors are centrally located, bilateral approach is very helpful for clear visualization of the neighboring structures in case of large sized tumors or those with local invasion (23). And surgeons need to be very familiar with the anatomy under VATS, from both the left and right approaches. During the process, it is always helpful to pull back the camera to re-orient to the anatomic perspective whenever one gets confused. As locally advanced lesions often involve more than one neighboring structure, it is advisable to start with the easiest part and then proceed gradually in an organized way during surgery. Multiple concomitant resections of involved structures were needed in these patients, including the lung, pericardium, phrenic nerve, and the innominate vein on one side, making it finally feasible to staple the lateral wall of the SVC. Last but not the least, it is critically important to bear in mind the oncological principles of VATS and make sure the tumor could be completely resected. Conversion to open surgery for oncological reasons should never be considered failure of VATS (20).
The other two patients in this series underwent right-sided VATS only, as their tumors were right-sided and invasion into the left thoracic cavity was not suspected before surgery. Although, in one of them tumor invasion into the left upper lobe was suspected during surgery, wedge resection was still able to be accomplished with the help of an additional subxyphoid port. Adding a subxyphoid port is also helpful during operation and removal of the resected sample when the tumor is of large size. This not only provides a better exposure, but also makes retrieval of the resected specimen much easier than through a rigid intercostal space and helps keeping the integrity of the tumor for histological examination.
The present study demonstrated that partial SVC resection via VATS was associated with acceptable operation time, small amount of blood loss, short duration of chest tube drainage and length of post-operative hospital stay. Morbidity is insignificant and there was no post-operative mortality. We presume that this procedure is safe and feasible in well-selected patients. As mentioned above, this procedure is technically highly demanding. Open surgery experience in controlling massive bleeding and in repairing or reconstructing vessels is necessary to ensure the safety of surgery. Besides, follow-up time for the current series was quite limited. Although no recurrence was detected in all 4 patients, the longest time after surgery in this series was only 14 months. Given the relative indolent nature of the disease, larger number of patients and longer follow-up time is needed to further assess the oncological efficacy of this procedure.
In conclusion, complete resection of locally advanced thymic tumors with limited invasion into the SVC can be accomplished via VATS. It is challenging but applicable in experienced hands. With gradual improvement in surgical techniques, more patients with locally advanced thymic tumors are expected to benefit from minimally invasive surgery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fang W, Yao X, Antonicelli A, et al. Comparison of surgical approach and extent of resection for Masaoka-Koga Stage I and II thymic tumours in Europe, North America and Asia: an International Thymic Malignancy Interest Group retrospective database analysis. Eur J Cardiothorac Surg 2017;52:26-32. [Crossref] [PubMed]
- Wang H, Gu Z, Ding J, et al. Members of the Chinese Alliance for Research in Thymomas. Perioperative outcomes and long-term survival in clinically early-stage thymic malignancies: video-assisted thoracoscopic thymectomy versus open approaches. J Thorac Dis 2016;8:673-9. [Crossref] [PubMed]
- Gu Z, Chen C, Wang Y, et al. Video-assisted thoracoscopic surgery versus open surgery for Stage I thymic epithelial tumours: a propensity score-matched study. Eur J Cardiothorac Surg 2018;54:1037-44. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta-analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Yang Y, Dong J, Huang Y. Thoracoscopic thymectomy versus open thymectomy for the treatment of thymoma: A meta-analysis. Eur J Surg Oncol 2016;42:1720-8. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-7. [Crossref] [PubMed]
- Tagawa T, Yamasaki N, Miyazaki T, et al. Thoracoscopic versus transsternal resection for early stage thymoma: long-term outcomes. Surg Today 2014;44:2275-80. [Crossref] [PubMed]
- Fadayomi AB, Iniguez CEB, Chowdhury R, et al. Propensity Score Adjusted Comparison of Minimally Invasive versus Open Thymectomy in the Management of Early Stage Thymoma. Thorac Cardiovasc Surg 2018;66:352-8. [Crossref] [PubMed]
- Agatsuma H, Yoshida K, Yoshino I, et al. Video-assisted thoracic surgery thymectomy versus sternotomy thymectomy in patients with thymoma Ann Thorac Surg 2017;104:1047-53. [Crossref] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology-Thymomas and Thymic Carcinomas (Version 2.2018) [EB/OL]. Available online: http://www.nccn.org.
- Chen KN, Xu SF, Gu ZD, et al. Surgical treatment of complex malignant anterior mediastinal tumors invading the superior vena cava. World J Surg 2006;30:162-70. [Crossref] [PubMed]
- Spaggiari L, Leo F, Veronesi G, et al. Superior vena cava resection for lung and mediastinal malignancies: a single center experience with 70 cases. Ann Thorac Surg 2007;83:223-9; discussion 229-30. [Crossref]
- Lanuti M, De Delva PE, Gaissert HA, et al. Review of superior vena cava resection in the management of benign disease and pulmonary or mediastinal malignancies. Ann Thorac Surg 2009;88:392-7. [Crossref] [PubMed]
- Leo F, Bellini R, Conti B, et al. Superior vena cava resection in thoracic malignancies: does prosthetic replacement pose a higher risk? Eur J Cardiothorac Surg 2010;37:764-9. [Crossref] [PubMed]
- Xu X, Qiu Y, Pan H, et al. Resection of the sidewall of superior vena cava using videoassisted thoracic surgery mechanical suture technique. J Thorac Dis 2016;8:612-6. [Crossref] [PubMed]
- Marx A, Chan JKC, Coindre JM, et al. The 2015 WHO Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an Evidence-Based Stage Classification System for the Forthcoming (8th) Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Burt BM, Yao X, Shrager J, et al. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Yao F, Wang R, Guo X, et al. Annual report of Department of Thoracic Surgery at Shanghai Chest Hospital. Shanghai Chest 2018;2:18. [Crossref]
- Toker A, Sonett J, Zielinski M, et al. Standard Terms, Definitions, and Policies for Minimally Invasive Resection of Thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Fang W, Feng J, Ji C, et al. Minimally invasive thymectomy for locally advanced recurrent thymoma. J Vis Surg 2016;2:58. [Crossref] [PubMed]
- Liu Q, Gu Z, Yang F, et al. The role of postoperative radiotherapy for stage I/II/III thymic tumor—results of the ChART retrospective database. J Thorac Dis 2016;8:687-95. [Crossref] [PubMed]
- Okumura M, Shintani Y, Funaki S, et al. VATS thymectomy—bilateral approach for extended resection. Mediastinum 2018;2:37. [Crossref]