
Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma
Introduction
Lung cancer is the most common cause of cancer-related death, responsible for nearly 1.8 million new cases diagnosed and 1.6 million deaths worldwide per year (1). Approximately 85% are non-small cell lung carcinoma (NSCLC), with more than 50% of adenocarcinoma and 30% of squamous cell carcinoma. Most patients present with advanced stage disease at the time of diagnosis (70%) and in the vast majority, only small samples, i.e., biopsies or cytology specimen, are available for diagnosis and biomarker testing. Less than half of lung adenocarcinoma harbor targeted driver EGFR, BRAF and HER2 mutations or ALK or ROS1 rearrangements, but in other NSCLC with no targeted molecular abnormalities the only therapeutic option was until recently conventional platinum-based doublet therapy, with pemetrexed maintenance for non-squamous NSCLC (2,3). This option offered a median overall survival of 1- and 5-year survival of 15%, all stages included. Since 2014, immunotherapies targeting programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) were evaluated in clinical trials, in monotherapy or in combination with chemotherapy or anti-CTLA4 agents, showing for a subset of patients a clear benefit with increased progression-free survival and overall survival. These immunotherapies are now available in routine practice and biomarkers predicting clinical response are complementary or mandatory for some drugs to better select patients who will benefit from immunotherapy.
Programmed cell death protein 1 (PD-1, or CD274 or B7-H1) is a co-stimulation receptor expressed by activated T cells, and binding to its ligands programmed death ligand 1 (PD-L1) or 2 (PD-L2) leads to a transient or permanent inhibition of CD8+ T cells cytotoxic properties. This interaction between receptor and ligands is physiological and controls autoimmunity, but when PD-L1 is engaged by tumor cells or immune cells, PD1+ CD8+T cells are inhibited, enabling the tumor to escape the adaptive anti-tumoral immune response. Hence, Immune Checkpoint Inhibitors (ICI) have been recently developed with the aim of restoring T cell cytotoxicity (4). They mainly target PD-1/PD-L1 axis and are represented by PD-1 inhibitors, such as nivolumab (OPDIVO®, Bristol-Myers Squibb) and pembrolizumab (KEYTRUDA®, Merck&Co), and PD-L1 inhibitors, such as atezolizumab (TECENTRIQ®, Genentech), durvalumab (IMFINZI®, Astra-Zeneca), and avelumab (BAVENCIO®, EMD Serono). Nivolumab and atezolizumab have been approved by the FDA and the EMA as second-line therapy in metastatic NSCLC irrespectively of PD-L1 expression. In contrast, the prescription of pembrolizumab for advanced NSCLC patients requires a companion diagnostic assay which is the demonstration by immunohistochemistry (IHC) of a minimum of 50% of PD-L1 positive tumor cells for first-line setting, and of 1% for second-line and beyond (5,6). Recently, the FDA approved durvalumab as maintenance therapy in patients with unresectable stage III NSCLC without progression after concurrent chemoradiotherapy.
PD-L1 expression in NSCLC tumors
PD-L1 is normally expressed by macrophages, some activated T cells and B cells, dendritic cells and some epithelial cells particularly in inflammatory conditions. Expression of PD-L1 by TC can be either innate or adaptative. Indeed, in a subset of NSCLC, PD-L1 expression is considered as constitutive, leading to an “innate immune resistance”, in relation with oncogenic alterations such as PD-L1 and JAK2 genomic amplification or PI3K/MAPK pathway activation (7,8). PD-L1 is often expressed by tumoral cells as an “adaptive resistance immune” mechanism, in order to escape anti tumoral response. This has been extensively explored and many biological processes lead to PD-L1 expression by tumoral cells. PD-L1 is related to an immune environment enriched in CD8+ T cells, with Th1 cytokines and chemokines production and with interferon γ gene expression signature. This immune environment has been described in “hot” tumors, contrasting with “cold” tumors where no immune cells could be found. Two main types of immune cells are associated with different clinical and biological characteristics. Neutrophils enriched tumors are significantly associated with STK11/LKB1 and KEAP1 mutations, a low PD-L1 expression, a low T-cell infiltration, and a primary resistance to PD-1 inhibitors (9-11). Conversely, CD8+ T cells enriched tumors are significantly associated with sarcomatoid carcinoma and adenocarcinoma with predominant solid pattern histology, TP53/ KRAS genes co-mutations, high levels of PD-L1 expression, activated interferon γ signaling pathway (including CD3, CD8, CD45RO, PD1, CTLA4 gene expression) and high mutational burden. Others factors may impact PD-L1 expression on tumoral cells, such as smoking status (positive correlation) (12), miRNA up (mir-20b, mir-21, mir-130b) or down regulation (mir-200, mir-197), hypoxia, epithelial mesenchymal transition (EMT) or as recently shown epigenomic mechanisms (13-19).
The multifaceted role or impact of PD-L1 expression on tumoral cells is the main reason for the ambivalent prognosis value of PD-L1 expression. It reflects a CD8+ T cells enriched environment reported to predict a better outcome (20,21), and some authors have proposed the ratio CD8+ T cells: T regs lymphocytes as predictive biomarker for ICI (22), but conversely, a high PD-L1 expression reflects tumor escape which can be targeted. The best situation for patients is probably to carry CD8+ T cells enriched tumors without PD-L1 expression by TC, ICI rescuing patients with PD-L1-positive NSCLC.
Nearly half of NSCLC exhibit PD-L1 membranous expression as defined by the Tumor Proportion Score (TPS; percentage of tumor cells with a linear membrane staining, at any intensity) ≥1%. Between 20% and 30% of NSCLC express high levels of PD-L1 (TPS ≥50%) and 30% a low to moderate PD-L1 expression (TPS between 1% and 49%) (Figure 1). This distribution seems relatively similar across ethnicities and countries, which is a little in contradiction with the fact that it could vary according to the main oncogenic driver alterations; in particular, lower prevalence of PD-L1 positivity was reported in EGFR mutated and ALK rearranged NSCLC in the EXPRESS study, a real-world multicentric study of PD-L1 expression prevalence in 2,634 stage IIB/IV NSCLC (23) and in the ATLANTIC trial (24). In addition, PD-L1 expression could also vary according to histological subtypes of NSCLC, type of samples, a prior chemo or radiotherapy, and according to trials, where different assays, criteria of positivity and thresholds were used (25-30).
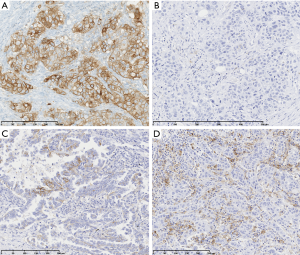
PD-L1 diagnostic tests
PD-L1 assays
Four standardized, FDA approved assays are commercially available to date, the Ventana PD-L1 SP263 assay (Ventana Medical Systems Inc.), the 28-8 PharmDx (Agilent Technologies/Dako) (31), the 22C3 PharmDx (Agilent Technologies/Dako) (32), and the Ventana PD-L1 SP142 Assay (Ventana Medical Systems Inc.) (33) (Table 1). All have been clinically validated in randomized trials for dedicated drugs. SP263 and 22C3 assays have been labeled as ‘companion’ diagnostic assays for pembrolizumab prescription by the FDA, the MHLW (Japanese Ministry of Health, Labour and Welfare), and the EMA and are CE-IVD marked, whereas the others are considered as complementary test. In second line setting, the 28-8 PharmDx assay and the Ventana PD-L1 SP263 assay have been approved by the FDA and the MHLW, and CE-IVD marked as a complementary diagnostic test for nivolumab. The Ventana PD-L1 SP142 assay was FDA cleared for atezolizumab as complementary diagnostic assay. The 73-10 PharmDx assay (Dako/Agilent Technologies) was developed to guide the prescription of avelumab and is FDA approved but the threshold of positivity has not been determined yet. Those assays differ in terms of primary monoclonal antibody, platform, detection system, and scoring with different thresholds of positivity validated in clinical trials. They all consider tumor cells membrane staining (partial or complete) whatever the intensity, except the SP142 assay which was developed for a scoring system taking into account either the percentage of PD-L1 expressing tumor cells (TC) and/or immune cells (IC), with for the latter a membrane or cytoplasmic staining.

Full table
Comparison of PD-L1 assays and platforms
Several studies have compared the different platforms and the different commercially available assays (Table 2). The phase I of the Blueprint PD-L1 IHC comparison project was conducted in collaboration between the International Association for the Study of Lung Cancer (IASLC), the American Association for Cancer Research (AACR), four pharmaceutical companies (Bristol-Myers Squibb, Merck, AstraZeneca and Genentech/Roche) and two diagnostic companies (Dako/Agilent and Ventana). The purpose was to evaluate the analytical performance of the 22C3 PharmDx and the 28-8 PharmDx on Autostainer Link 48 platform (Agilent Technologies, Santa Clara, CA, USA) and the Ventana PD-L1 SP263 and SP142 Assays on the Ventana ULTRA platform (Ventana medical Systems, Tucson, AZ, USA) in a series of 38 NSCLC surgical samples (34). Stainings were analyzed centrally by 3 trained pathologists from the Ventana/Roche and Dako/Agilent diagnostic companies. Each assay was analyzed using the threshold and the scoring method retained in the corresponding clinical trials [≥1% tumor cell staining for the 28-8 and 22C3 assays, ≥25% tumor cell staining for the SP263 assay and ≥1% tumor cell staining and ≥1% tumor area infiltrated by PD-L1-positive immune cells (TC1/IC1) for the SP142 assay]. There was no training or pre-alignment between pathologists before the study. The conclusions were that 22C3, 28-8, and SP263 assays harbored similar analytical performance for the tumor cells staining and with a good inter-observers’ concordance (overall percentage of agreement OPA >85%), but for 37% of the cases, a misclassification of PD-L1 status was made depending on the assays and thresholds used. In addition, the concordance was poor for immune cells assessment with a great variability between staining and scores. The German harmonization study (35) confirmed the good performance of 22C3 PharmDx, 28-8 PharmDx and SP263 Assay on a series of 15 resected NSCLC evaluated by 9 pathologists. Good concordance coefficients (κ value=0.6–0.8) were demonstrated for ≥1, ≥5, ≥10, ≥50% TPS categories, and again, all but the SP142 assay, provided high percentage of agreement between observers, with poor agreement (kappa value around 0.2) for immune cells. Interestingly, the authors showed that SP263 assay stained more tumor cells, and SP142 and SP263 stained immune cells more intensely. The NCCN study by Rimm et al. analyzed 90 resected NSCLC stained by 28.8 and 22C3 PharmDx and SP142 assays and assessed by 13 pathologists (36). This study reached the same conclusions with interclass coefficient correlation for TPS and immune cells proportion score of 0.813 (95% CI, 0.815–0.839) and 0.277 (95% CI, 0.222–0.334), respectively. Accordingly, Ratcliffe et al. (37) stained a series of 493 NSCLC with 22C3 and 28-8 PharmDx and SP263 assays analyzed by a single pathologist from a clinical laboratory improvement amendments (CLIA) certified laboratory, and showed OPA >90% at different cut-offs. At least, the Blueprint phase 2A project compared the 5 assays (22C3 28-8, SP142, SP263 and 73-10) (38) in a large series of 80 samples including biopsies and cytological specimen, and reported a higher sensitivity of the 73-10 assay to detect PD-L1 expression on tumor cells. A high concordance between scanned slides digital pathology vs. glass slides was also observed. Recently, it has been suggested that SP263 may stain a higher proportion of tumor cells as compared to the 22C3 assay (39), challenging the interchangeability between these 2 assays. Interestingly, in most following studies, a better agreement was obtained among pathologists with a TPS ≥50%, but the variability between pathologists for a given assay was often higher than between the assays (40,41).
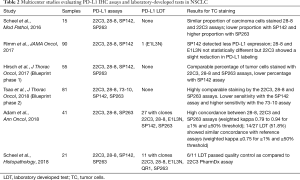
Full table
Laboratory developed tests
Since Dako and Ventana IHC platforms are not available in all laboratories and given the high costs of the standardized, ready-to-use, PD-L1 assays, and the small size of the NSCLC samples precluding using a different assay for each specific drug, a growing number of pathologists have implemented PD-L1 laboratory-developed tests. These tests were developed more frequently with concentrated antibodies (mainly 22C3, 28-8, E1L3N and QR1), and rarely with prediluted antibodies retrieved from the PD-L1 assays to be used in non-dedicated platforms. The two main studies comparing LDT were performed by cooperative groups in France and Germany. The French group of thoracic pathologists (PATTERN) has conducted a multicentric study (42) to compare LDT with 22C3, 28-8, SP142, SP263, and E1L3N antibodies on Ventana BenchMark Ultra, Bond (Leica Biosystems) or Autostainer Link 48 (Dako/Agilent). The stainings were performed in 7 centers on a series of 41 surgical samples of NSCLC. Among the 27 LDT tested, only 14 (51.8%) demonstrated sufficient concordance (weighted kappa ≥0.75 for the ≥1% and ≥50% thresholds) as compared to reference assays for tumor cell staining. Clone SP263 achieved the highest concordance rate across all platforms, with a percentage of agreement of more than 90% on all platforms considering the 1% and 50% thresholds. These data are in agreement with the German study (43) where eleven LDT using 22C3, 28-8, QR1, SP263, and E1L3N antibodies were tested on the same three platforms, with only 54% (6 out of 11) passing the quality control tests. Some studies have specifically evaluated LDT with 22C3 clone and validated protocols to be used either on Ventana BenchMark Ultra, Bond III (Leica Biosystems) or DAKO Omnis autostainers (44-46) offering a 85% to 100% concordance between LDT on different platforms and 22C3 PharmDx assay on 48 Link Dako/Agilent autostainer (Table 2).
Practical considerations for test implementation in routine
Samples
PD-L1 expression is heterogeneous in a significant proportion of NSCLC surgical specimens, when comparing small regions (microscopic fields from one FFPE block) (47), but this heterogeneity is rather constant between large regions, when comparing several blocks from the same case. Rehman et al. have observed a high correlation of 94% for PD-L1 TC expression and 75% for immune cells scores using the SP142 assay between 3 blocks of the same tumor (48). The intra tumoral heterogeneity is well known from pathologists as it represents a serious issue to score PD-L1 expression from large tissue specimens. It also questions the representativeness of small samples, i.e., biopsies or cytology specimen, which represent approximately 70% of the specimen for lung cancer diagnosis. Several studies have reported various results regarding the concordance between paired biopsies and surgical samples. In a series of 160 NSCLC, Ilie et al. have shown a 48% discordance rate between biopsies and paired surgical resection, using the SP142 assay and the TC/IC scoring scale developed for atezolizumab trials. In particular, immune cells at the periphery of the tumor were less represented on biopsies and accounted for a significant proportion of discordances (49). Two others studies using various primary antibodies and evaluating PD-L1 expression in TC only, have found high concordance rates, as high as 92% between small biopsies and resected specimen (50,51). Tumoral heterogeneity could be probably better assessed using image analysis and staining intensity quantification by fluorescence [quantitative immunofluorescent (QIF)] but those methods are not yet available in the routine pathology setting to date.
PD-L1 expression may vary over time during tumor progression and between primary and metastatic locations. Kim et al. have shown a concordance of only 75% between primitive tumor and node metastasis regarding PD-L1 expression whatever the thresholds of 1%, 5%, 10% and 50% (52). In contrast, Liu et al. (53) have shown a concordance of 90% and 78% respectively between primary squamous and adenocarcinoma and their node metastasis for PD-L1 expression by TC ≥1%. Regarding visceral metastasis, a concordance of 78% was observed between paired primary lung tumors and resected metastasis, with a rate of 11% of primary positive tumor/metastatic negative tumor or vice versa (54) and in the ATLANTIC trial, a concordance of 89% was observed between primary and metastatic samples (35% and 33% of positive cases, respectively) (55). Interestingly in the EXPRESS study, no difference was observed in term of prevalence of PD-L1 expression ≥50% or ≥1% between primary (n=1,735) and metastatic tumors (n=565) (23). Apparently, the major discordances are observed when samples are obtained with more than 6 months of interval (56). Taken together, current data suggest that primary site or metastasis can be both biopsied and used for PD-L1 assessment provided that the sample is taken freshly before the patient’s treatment by ICI.
PD-L1 expression seems to be modified by conventional chemotherapy, radiotherapy and tyrosine kinase inhibitors (57). Most series demonstrated an increase of PD-L1 expression after chemotherapy and radiotherapy, particularly when platinum-based regimen was used, but some other works reported a decrease of PD-L1 expression after neoadjuvant chemotherapy (58-63). In the KEYNOTE-010 trial evaluating pembrolizumab in pretreated metastatic NSCLC patients, there was no difference in terms of PD-L1 expression between archival samples (before chemotherapy) and fresh biopsies (after chemotherapy) (29). Cho et al. has reported a concordance of 67% regarding PD-L1 expression between samples before and after chemotherapy (64). The use of tyrosine kinase inhibitors including osimertinib, could decrease the level of PD-L1 expression in EGFR mutated tumors (65,66), but conversely, when those tumors acquire gefitinib resistance, they seem to express more strongly PD-L1 (67). Such discrepancies in the results highlight the difficulty in differentiating the specific effect of treatment and spatial heterogeneity when comparing small samples collected before and after treatment.
In practice, the rational use of small samples is desirable: (I) by encouraging the inclusion of fragments in several cassettes, (II) by immediately preparing unstained slides to avoid cutting the block again. This includes sufficient slides for diagnostic and theranostic IHC (ALK, ROS1 and PD-L1) and fluorescent in situ hybridization (FISH) and for molecular analyses for non-squamous NSCLC. PD-L1 IHC must be performed systematically in case of stage III–IV NSCLC without waiting for the clinician’s prescription, in order to allow rapid therapeutic decision. It can be performed a posteriori in case of disease progression at the request of the clinician.
A minimum of 100 analyzable tumor cells is required for PD-L1 interpretation in the 28-8 and 22C3 PharmDx interpretation manuals (Agilent/Dako) (68,69). When the number of tumor cells is less than 100 and especially less than 50, our group has recommended to report the result to the clinician with limitations regarding the representativeness of the sample (70). Ninety-five percent of biopsies are adequate for PD-L1 testing with comparable results between biopsies and surgical specimen when they contain more than 100 tumor cells (71). Fixed and paraffin-embedded cytological specimens (cytoblocks) from endobronchial ultrasound guided biopsies (EBUS), transesophageal ultrasound guided biopsies (EUS), pleural fluid punctures and transthoracic aspiration punctures (TBNA) may be used for PD-L1 expression evaluation when these specimens contain at least 100 analyzable tumors cells. Nevertheless, the use of cytological specimens has not been validated in clinical trials and is not currently recommended for use in PD-L1 assays. However, several studies have shown good agreement between cell blocks, biopsies or surgical specimens (38,71-73). Skov et al. have shown that discrepancies between histological samples and cytological samples were mainly related to tumor heterogeneity in biopsies particularly with PD-L1 expression cut-offs of 5% and 10% (72).
The pre-analytical phase conditions the quality of the subsequent techniques (IHC, ISH and Molecular Biology) and thus the detection of PD-L1 by IHC (74,75). Studies dedicated to PD-L1 IHC have not evaluated the effect of cold ischemia delay on its expression but this has been extensively shown for other markers including hormone receptors and HER2 in breast cancers (76,77). Samples should be fixed in 10% buffered neutral formaldehyde for 6 to 48 hours, using a sufficient volume of fixative. The only fixative recommended is neutral formalin buffered at 10%, as the protocols have been clinically validated only for formalin fixation. Cytological samples used in different studies for the expression of PD-L1 were fixed either with buffered formalin or with Cytolyt® (methanolic fixative, Hologic) but to date, no standardized PD-L1 test has been validated for the use after CytoLyt® fixation. Regarding decalcification, in general it should be avoided as much as possible because it is often harmful for HC, ISH, and molecular biology, as it alters nucleic acids and proteins. Moreover, the various standardized tests have not been validated for decalcified samples (68,69,78).
Validation of tests
Given the large variability in expression of PD-L1 in NSCLC (percentage and staining intensity), calibration and validation of the technique is recommended, particularly when PD-L1 IHC is performed with a LDT. As general considerations, laboratories must validate any IHC before placing into clinical service, by correlating the new test with expected results (clinically and morphologically) and comparing the new results on samples already tested in a phase III trial or if not possible with results obtained with a prior validated assay and with the results obtained in another laboratory on same samples. In addition, continuous quality monitoring and participation to External Quality Assurance (EQA) programmes are highly recommended (79). For theranostic tests, a sample validation set must be chosen including at least 20 positive and 20 negative cases, and the positive cases should span the range of clinical results (80). In practice, the validation set for PD-L1 IHC should include tumor samples with more than 50% of positive tumor cells, with 1–49% of positive tumor cells and less than 1%. This set can be enriched in samples with an epitope concentration close to the thresholds of positivity of the clinically validated assays and those samples have to be handled similar to clinical specimens regarding fixative and processing methods. Ideally, the evaluation of the test includes an assessment of its sensitivity and specificity, of its inter-run and inter-operator variability, as well as a calculation of positive and negative concordance rates, with an expected 90% overall concordance versus reference test [similar to a weighted kappa value >0.75 in our study for each of the thresholds used in the clinic (42)]. In addition, it is advisable to regularly record the number of negative, weakly positive or strongly positive cases to ensure the number of negative tumors does not exceed 50% of all samples and that tumors with a TPS ≥50% represent at least 20% of all cases, in agreement with the PD-L1 expression prevalence reported in the literature. To ensure the reproducibility of the IHC technique, positive and negative external controls should be included on each slide as far as possible; otherwise an external control slide should be provided for each run. Tumors (whole sections or TMA) managed under the same conditions as the samples can be used as external controls. Cell lines presenting with variable PD-L1 intensity are now commercially available. Their PD-L1 expression is stable and homogeneous, allowing a better standardization of the technique. Conversely, normal tonsils and placental tissue (trophoblastic cells) have to be use with caution due to the very high expression level of PD-L1 by epithelial cells and trophoblastic cells, respectively, which may conceal a lack of sensitivity of the technique. Ideally, the controls should be cut at the same time as the sample, but unstained slides can be prepared in advance, respecting storage time and conditions recommendations. At least, a minimum number of tests must be carried out and interpreted per year in each pathology structure to establish technical and medical expertise and optimize the quality and costs of analyses.
Interpretation: influence of training
Any quantitative or semi-quantitative interpretation of an immunohistochemical analysis is subject to some variability between readers. PD-L1 TC staining assessment by pathologists in NSCLC may be difficult, in particular because of the low intensity of staining in some cases, the high intra tumoral heterogeneity and the staining of immune cells. Despite these potential issues, several studies have found a moderate to strong interobserver agreement for PD-L1 assessment using TPS score, with discrepancies rates for ≥1% and ≥50% thresholds close to 10% (Table 3). Discrepancies in the interpretation of PD-L1 staining could be related to disparities in the training of pathologists, but in the DREAM intra- and inter-observer reproducibility study, where 60 NSCLC were analyzed by 10 pathologists, the OPA values for intra-observer reproducibility for ≥1% and ≥50% cut offs were very high (90% and 91%, respectively), whereas the inter-observer reproducibility was lower (82% and 78%), respectively, but slightly improved after training for ≥50% cutoff (82%) (81). However, in this study training was limited to 1 hour and the impact of more intensive training programs has not been reported.

Full table
Reporting of PD-L1 IHC
The report of the PD-L1 IHC in NSCLC must mention the date, the site, the type of sample (cytology, biopsy, and surgical samples), the fixative, a possible decalcification, as well as some parameters that may influence PD-L1 expression such as disease stage, the notion of primary tumor, recurrence or metastasis, or previous treatments (70,82). It may incorporate genomic alterations known to be associated more frequently with high PD-L1 expression, even if this information is usually not available in case of upfront PD-L1 testing. For the analytical part, it is necessary to indicate the antibody (clone) and the autostainer used, as well as to specify if it is an assay or a LDT. Other indications such as antibody dilution and incubation time are not essential. For the analytical part, only the percentage of stained tumors cells, whatever the intensity, should be reported. It is necessary to mention the use of positive external and/or internal controls (immune cells) and for small samples, to specify how many tumor cells were analyzed, with limitations if less than 100, and if the pre-analytical stages were problematic (fixation unknown or out of time, decalcification, too long storage time of unstained slides, etc.). At present, there is no clinical utility in the evaluation of PD-L1 expression by immune cells for the prescription of ICI. As regards the conclusion of the report, it is preferable to simply indicate the percentage of tumor cells expressing PD-L1. Indications regarding eligibility for certain immunotherapies are not recommended as the therapeutic strategy for immunotherapy is constantly evolving and depends on many other clinical parameters.
Quality assurance (QA)
Like any theranostic test, PD-L1 immunohistochemical test must be subjected to validation and robust internal and external quality controls. The PD-L1 test must be carried out subject to participation in an external quality assessment with satisfactory results, according to the criteria defined by the QA guidelines. Several external quality assurance studies have evaluated PD-L1 testing and emphasized the use of QA programs. The Nordic QC program showed in 2017 that in 37 pathology laboratories using either 28.8 or 22C3 PharmDx or SP263 assay, 69% to 92% of the stains were considered as optimal to good, whereas in the 29 labs using LDT, less than on third gave optimal staining (83). In May 2018, LDT using E1L3N or 22C3 Abs gave sufficient staining in 100% and 97%, respectively (84). The UK NEQAS PD-L1 pre-pilot EQA also showed 53% of LDT (n=19) exhibited unacceptable results, contrasting with assays which gave in 71% of the tests (n=24), acceptable staining (85). Recent international recommendations (80,86) call for a quality control program for analytical techniques in laboratories performing theranostic tests, with particular emphasis on the use of calibrated controls for each set of tests.
Conclusions
Immunohistochemical evaluation of PD-L1 expression by tumor cells is now required for the first- and second-line prescription of pembrolizumab in metastatic non-small cell lung cancers and in stage III non resectable NSCLC after chemoradiotherapy for durvalumab. This test must therefore be implemented in all laboratories dealing with lung cancer diagnosis, and performed at the same time as the other theranostic markers. Various studies have shown the reliability of the immunohistochemical technique when performed with clinically validated commercial tests or kits and the good reproducibility of pathologists for the quantification of labelling when they are trained. The use of so-called in-house protocols is possible, but it is our responsibility to ensure that the technical conditions and analyses of the results are reliable and reproducible and to further harmonize our practices and strengthen the quality of this theranostic test to further improve patient management.
Acknowledgements
None.
Footnote
Conflicts of Interest: S Lantuejoul declares consultancy and/or honoraria (speaker) for AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharp & Dohme, Roche, Ventana; J Adam declares consultancy and/or honoraria (speaker) for AstraZeneca, Bristol-Myers Squibb, Roche, Merck Sharp & Dohme; V Hofman declares no conflict of interest; D Damotte declares consultancy and/or honoraria (speaker) Bristol-Myers Squibb, AstraZeneca, Merck Sharp & Dohme, and Roche.
References
- American Cancer Society. Global cancer facts & figures. 3rd ed. Atlanta: American Cancer Society; 2015.
- Masters GA, Temin S, Azzoli CG, et al. Systemic Therapy for Stage IV Non–Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015;33:3488-515. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Harvey RD. Immunologic and Clinical Effects of Targeting PD-1 in Lung Cancer. Clin Pharmacol Ther 2014;96:214-23. [Crossref] [PubMed]
- Duruisseaux M, Rouquette I, Adam J, et al. Efficacy of PD-1/PD-L1 immune checkpoint inhibitors and PD-L1 testing in thoracic cancers. Ann Pathol 2017;37:61-78. [Crossref] [PubMed]
- Granier C, Soumelis V, Mandavit M, et al. The “immune checkpoints”, how does it work. Ann Pathol 2017;37:18-28. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Clavé S, Pijuan L, Casadevall D, et al. CD274 (PDL1) and JAK2 genomic amplifications in pulmonary squamous-cell and adenocarcinoma patients. Histopathology 2018;72:259-69. [Crossref] [PubMed]
- Skoulidis F, Byers LA, Diao L, et al. Co-occurring Genomic Alterations Define Major Subsets of KRAS-Mutant Lung Adenocarcinoma with Distinct Biology, Immune Profiles, and Therapeutic Vulnerabilities. Cancer Discov 2015;5:860-77. [Crossref] [PubMed]
- Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS -Mutant Lung Adenocarcinoma. Cancer Discov 2018;8:822-35. [Crossref] [PubMed]
- Biton J, Mansuet-Lupo A, Pécuchet N, et al. TP53, STK11, and EGFR Mutations Predict Tumor Immune Profile and the Response to Anti–PD-1 in Lung Adenocarcinoma. Clin Cancer Res 2018;24:5710-23. [Crossref] [PubMed]
- Koh J, Go H, Keam B, et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod Pathol 2015;28:1154-66. [Crossref] [PubMed]
- Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018;48:434-52. [Crossref] [PubMed]
- Ribas A. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov 2015;5:915-9. [Crossref] [PubMed]
- Zhu J, Chen L, Zou L, et al. MiR-20b, -21, and -130b inhibit PTEN expression resulting in B7-H1 over-expression in advanced colorectal cancer. Hum Immunol 2014;75:348-53. [Crossref] [PubMed]
- Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother 2007;30:251-60. [Crossref] [PubMed]
- Ikeda S, Okamoto T, Okano S, et al. PD-L1 Is Upregulated by Simultaneous Amplification of the PD-L1 and JAK2 Genes in Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:62-71. [Crossref] [PubMed]
- Barsoum IB, Smallwood CA, Siemens DR, et al. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res 2014;74:665-74. [Crossref] [PubMed]
- Duruisseaux M, Martínez-Cardús A, Calleja-Cervantes ME, et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: a multicentre, retrospective analysis. Lancet Respir Med 2018;6:771-81. [Crossref] [PubMed]
- Goc J, Germain C, Vo-Bourgais TKD, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014;74:705-15. [Crossref] [PubMed]
- Brambilla E, Le Teuff G, Marguet S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:1223-30. [Crossref] [PubMed]
- Wu SP, Liao RQ, Tu HY, et al. Stromal PD-L1–Positive Regulatory T cells and PD-1–Positive CD8-Positive T cells Define the Response of Different Subsets of Non–Small Cell Lung Cancer to PD-1/PD-L1 Blockade Immunotherapy. J Thorac Oncol 2018;13:521-32. [Crossref] [PubMed]
- Dietel M, Savelov N, Salanova R, et al. 130O Real-world prevalence of PD-L1 expression in locally advanced or metastatic non-small cell lung cancer (NSCLC): The global, multicentre EXPRESS study. J Thorac Oncol 2018;13:S74-5. [Crossref]
- Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018;19:521-36. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Phillips T, Simmons P, Inzunza HD, et al. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl Immunohistochem Mol Morphol 2015;23:541-9. [Crossref] [PubMed]
- Dolled-Filhart M, Locke D, Murphy T, et al. Development of a Prototype Immunohistochemistry Assay to Measure Programmed Death Ligand-1 Expression in Tumor Tissue. Arch Pathol Lab Med 2016;140:1259-66. [Crossref] [PubMed]
- Vennapusa B, Baker B, Kowanetz M, et al. Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl Immunohistochem Mol Morphol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Scheel AH, Dietel M, Heukamp LC, et al. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod Pathol 2016;29:1165-72. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-8. [Crossref] [PubMed]
- Ratcliffe MJ, Sharpe A, Midha A, et al. Agreement between Programmed Cell Death Ligand-1 Diagnostic Assays across Multiple Protein Expression Cutoffs in Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:3585-91. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J Thorac Oncol 2018;13:1302-11. [Crossref] [PubMed]
- Munari E, Rossi G, Zamboni G, et al. PD-L1 Assays 22C3 and SP263 are Not Interchangeable in Non-Small Cell Lung Cancer When Considering Clinically Relevant Cutoffs: An Interclone Evaluation by Differently Trained Pathologists. Am J Surg Pathol 2018;42:1384-9. [Crossref] [PubMed]
- Marchetti A, Barberis M, Franco R, et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J Thorac Oncol 2017;12:1654-63. [Crossref] [PubMed]
- Brunnström H, Johansson A, Westbom-Fremer S, et al. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: inter-pathologist variability is higher than assay variability. Mod Pathol 2017;30:1411-21. [Crossref] [PubMed]
- Adam J, Le Stang N, Rouquette I, et al. Multicenter French harmonization study for PD-L1 IHC testing in non-small cell lung cancer. Ann Oncol 2018;29:953-8. [Crossref] [PubMed]
- Scheel AH, Baenfer G, Baretton G, et al. Interlaboratory concordance of PD-L1 immunohistochemistry for non-small-cell lung cancer. Histopathology 2018;72:449-59. [Crossref] [PubMed]
- Neuman T, London M, Kania-Almog J, et al. A Harmonization Study for the Use of 22C3 PD-L1 Immunohistochemical Staining on Ventana’s Platform. J Thorac Oncol 2016;11:1863-8. [Crossref] [PubMed]
- Ilie M, Khambata-Ford S, Copie-Bergman C, et al. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS One 2017;12:e0183023. [Crossref] [PubMed]
- Røge R, Vyberg M, Nielsen S. Accurate PD-L1 Protocols for Non–Small Cell Lung Cancer can be Developed for Automated Staining Platforms With Clone 22C3 Appl Immunohistochem Mol Morphol 2017;25:381-5. [Crossref] [PubMed]
- McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. [Crossref] [PubMed]
- Rehman JA, Han G, Carvajal-Hausdorf DE, et al. Quantitative and pathologist-read comparison of the heterogeneity of programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer. Mod Pathol 2017;30:340-9. [Crossref] [PubMed]
- Ilie M, Long-Mira E, Bence C, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol 2016;27:147-53. [Crossref] [PubMed]
- Gradecki SE, Grange JS, Stelow EB. Concordance of PD-L1 Expression Between Core Biopsy and Resection Specimens of Non-Small Cell Lung Cancer. Am J Surg Pathol 2018;42:1090-4. [Crossref] [PubMed]
- Kitazono S, Fujiwara Y, Tsuta K, et al. Reliability of Small Biopsy Samples Compared With Resected Specimens for the Determination of Programmed Death-Ligand 1 Expression in Non--Small-Cell Lung Cancer. Clin Lung Cancer 2015;16:385-90. [Crossref] [PubMed]
- Kim MY, Koh J, Kim S, et al. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 2015;88:24-33. [Crossref] [PubMed]
- Liu Y, Dong Z, Jiang T, et al. Heterogeneity of PD-L1 Expression Among the Different Histological Components and Metastatic Lymph Nodes in Patients With Resected Lung Adenosquamous Carcinoma. Clin Lung Cancer 2018;19:e421-30. [Crossref] [PubMed]
- Kim HR, Cha YJ, Hong MH, et al. Concordance of programmed death-ligand 1 expression between primary and metastatic non-small cell lung cancer by immunohistochemistry and RNA in situ hybridization. Oncotarget 2017;8:87234-43. [PubMed]
- Midha A, Sharpe A, Scott M, et al. PD-L1 expression in advanced NSCLC: Primary lesions versus metastatic sites and impact of sample age. J Clin Oncol 2016;34:3025. [Crossref]
- Mansfield AS, Aubry MC, Moser JC, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol 2016;27:1953-8. [Crossref] [PubMed]
- Kao CJ, Wurz GT, Lin YC, et al. Assessing the Effects of Concurrent versus Sequential Cisplatin/Radiotherapy on Immune Status in Lung Tumor-Bearing C57BL/6 Mice. Cancer Immunol Res 2015;3:741-50. [Crossref] [PubMed]
- Haratake N, Toyokawa G, Tagawa T, et al. Positive Conversion of PD-L1 Expression After Treatments with Chemotherapy and Nivolumab. Anticancer Res 2017;37:5713-7. [PubMed]
- Funaki S, Shintani Y, Kawamura T, et al. Chemotherapy enhances programmed cell death 1/ligand 1 expression via TGF-β induced epithelial mesenchymal transition in non-small cell lung cancer. Oncol Rep 2017;38:2277-84. [Crossref] [PubMed]
- Zhang P, Ma Y, Lv C, et al. Upregulation of programmed cell death ligand 1 promotes resistance response in non-small-cell lung cancer patients treated with neo-adjuvant chemotherapy. Cancer Sci 2016;107:1563-71. [Crossref] [PubMed]
- Song Z, Yu X, Zhang Y. Altered expression of programmed death-ligand 1 after neo-adjuvant chemotherapy in patients with lung squamous cell carcinoma. Lung Cancer 2016;99:166-71. [Crossref] [PubMed]
- Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep 2016;6:20090. [Crossref] [PubMed]
- Rojkó L, Reiniger L, Téglási V, et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J Cancer Res Clin Oncol 2018;144:1219-26. [Crossref] [PubMed]
- Cho JH, Sorensen SF, Choi YL, et al. Programmed Death Ligand 1 Expression in Paired Non-Small Cell Lung Cancer Tumor Samples. Clin Lung Cancer 2017;18:e473-9. [Crossref] [PubMed]
- Lin K, Cheng J, Yang T, et al. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun 2015;463:95-101. [Crossref] [PubMed]
- Jiang XM, Xu YL, Huang MY, et al. Osimertinib (AZD9291) decreases programmed death ligand-1 in EGFR-mutated non-small cell lung cancer cells. Acta Pharmacol Sin 2017;38:1512-20. [Crossref] [PubMed]
- Han JJ, Kim DW, Koh J, et al. Change in PD-L1 Expression After Acquiring Resistance to Gefitinib in EGFR-Mutant Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016;17:263-270.e2. [Crossref] [PubMed]
- PD-L1 IHC 28-8 pharmDx. Available online: https://www.agilent.com/cs/library/usermanuals/public/29111_pd-l1-ihc-28-8-interpretation-manual.pdf
- PD-L1 IHC 22C3 pharmDx is CE-IVD–marked For In Vitro Diagnostic Use. Available online: https://www.agilent.com/cs/library/usermanuals/public/29171_22C3-ihc-pharmdx-interpretation-manual-eu.pdf
- Lantuejoul S, Adam J, Girard N, et al. PD-L1 testing in non-small cell lung carcinoma: Guidelines from the PATTERN group of thoracic pathologists. Ann Pathol 2018;38:110-25. [Crossref] [PubMed]
- Heymann JJ, Bulman WA, Swinarski D, et al. Programmed death-ligand 1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol 2017;125:896-907. [Crossref] [PubMed]
- Skov BG, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx Appl Immunohistochem Mol Morphol 2017;25:453-9. [Crossref] [PubMed]
- Aisner DL, Rumery MD, Merrick DT, et al. Do More With Less: Tips and Techniques for Maximizing Small Biopsy and Cytology Specimens for Molecular and Ancillary Testing: The University of Colorado Experience. Arch Pathol Lab Med 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Engel KB, Moore HM. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med 2011;135:537-43. [PubMed]
- Bass BP, Engel KB, Greytak SR, et al. A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: how well do you know your FFPE specimen? Arch Pathol Lab Med 2014;138:1520-30. [Crossref] [PubMed]
- Khoury T. Delay to formalin fixation alters morphology and immunohistochemistry for breast carcinoma. Appl Immunohistochem Mol Morphol 2012;20:531-42. [Crossref] [PubMed]
- Bussolati G, Annaratone L, Maletta F. The pre-analytical phase in surgical pathology. Recent Results Cancer Res 2015;199:1-13. [Crossref] [PubMed]
- Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160006C.pdf
- Fitzgibbons PL, Goldsmith JD, Souers RJ, et al. Analytic Validation of Immunohistochemical Assays: A Comparison of Laboratory Practices Before and After Introduction of an Evidence-Based Guideline. Arch Pathol Lab Med 2017;141:1247-54. [Crossref] [PubMed]
- Torlakovic EE, Cheung CC, D’Arrigo C, et al. Evolution of Quality Assurance for Clinical Immunohistochemistry in the Era of Precision Medicine. Part 3: Technical Validation of Immunohistochemistry (IHC) Assays in Clinical IHC Laboratories. Appl Immunohistochem Mol Morphol 2017;25:151-9. [Crossref] [PubMed]
- Cooper WA, Russell PA, Cherian M, et al. Intra- and Interobserver Reproducibility Assessment of PD-L1 Biomarker in Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:4569-77. [Crossref] [PubMed]
- IASLC Atlas of PD-L1 immunohistochemistry testing in lung cancer. Available online: https://www.iaslc.org/sites/default/files/wysiwyg-assets/iaslc_pd-l1_atlas_mar2018_lo-res.pdf
- Available online: http://www.nordiqc.org/downloads/assessments/96_102.pdf
- Available online: http://www.nordiqc.org/downloads/assessments/108_102.pdf
- Available online: http://www.ukneqasiccish.org/wp/wp-content/uploads/2017/09/PD-L1_prepilot_write_up_070917.pdf
- Torlakovic EE, Cheung CC, D’Arrigo C, et al. Evolution of Quality Assurance for Clinical Immunohistochemistry in the Era of Precision Medicine - Part 2: Immunohistochemistry Test Performance Characteristics. Appl Immunohistochem Mol Morphol 2017;25:79-85. [Crossref] [PubMed]


