
Galactomannan detection in bronchoalveolar lavage fluid corrected by urea dilution for the diagnosis of invasive pulmonary aspergillosis among nonneutropenic patients
Introduction
Invasive pulmonary aspergillosis (IPA) is a serious filamentous fungal respiratory infection that causes high mortality and morbidity. Previously, IPA was considered to appear mostly in patients with neutropenia, such as haematopoietic stem cell transplantation (HSCT) patients. Currently, a rising incidence has been observed in nonneutropenic hosts, most of whom are admitted to an intensive care unit (ICU) (1).
Accurately diagnosing of IPA in ICU patients is quite challenging, because the symptoms and signs are nonspecific, subtle and unable to be distinguished from those of bacterial pneumonia or even non-infectious diseases (2). Conventional culture methods are considered to have significant importance for identifying Aspergillus spp., although positive results are obtained for only approximately 50% of patients with IPA (3). The AspICU clinical algorithm (listed in Supplementary file 1) has been proposed to discriminate IPA in ICU patients due to its relatively high diagnostic utility (4). However, this clinical algorithm includes at least one positive Aspergillus culture in a respiratory tract specimen as an inclusion criterion, which leads to misdiagnosis or missed diagnosis of IPA patients with negative culture results.
Galactomannan (GM) detection in bronchoalveolar lavage fluid (BALF) is recommended in the practice guidelines for IPA diagnosis due to its higher sensitivity and specificity than its detection in serum. However, the optimal cut-off point for GM in BALF samples for the diagnosis of IPA has not been unified due to the use of varying dilutions. An ODI of 1.0 was recommended by the Infectious Diseases Society of America (IDSA) for clinical testing, whereas an ODI of 0.5 to 1.0 was applied in the new aspergillosis disease guideline of the ESCMID-ECMM-ERS (5,6). Urea is a diffusible substance that can easily be detected in all body compartments, including capillaries and the alveolar space (7), and the urea concentrations in both compartments are maintained at a constant ratio (8). The ratio of the plasma to BALF urea concentration was previously applied as an index of the epithelial lining fluid (ELF) dilution, and the real concentrations of antibiotics in the ELF were corrected by urea dilution in many pharmacokinetics studies (9,10).
These theories led to the hypothesis that urea dilution could be used as an index to correct the real GM concentration in the BALF. Therefore, the present study aimed to investigate the optimal cut-off point of a corrected BALF GM value and to determine whether the modified AspICU clinical algorithm had a better diagnostic performance when based upon the corrected concentration.
Methods
Research briefs
This multi-centre, prospective, observational study was performed in the Department of Critical Care Medicine in three teaching hospitals in Shanghai that accounted for a total of 155 ICU hospital beds. The annual volume of patients admitted to these three ICUs was approximately 3,200. The three participating units were Renji Hospital, Ruijin Hospital (both affiliated with Shanghai Jiao Tong University School of Medicine) and Minhang District Central Hospital (affiliated with Fudan University School of Medicine). This study was approved by the Ethics Committee of Shanghai Jiao Tong University (No. 2016-Clinical-Res-083), and informed written consent was obtained from either the patients or their next of kin.
Study population
Patients admitted to the ICU with suspected IPA (possible diagnosis) were enrolled in our study from January 2016 to June 2018. Patients were included if they met the following criteria: (I) peripheral blood neutrophil count >0.5×109/L; (II) BALF and serum GM detection tests were performed; (III) other necessary differential diagnostic tests, such as chest computed tomography (CT) and lower respiratory tract specimen culture, were performed; (IV) urea was tested in both plasma and BALF samples; and (V) age between 18 and 80 years. Patients were excluded from the study if the following criteria were met: (I) they received antifungal treatment prior to the GM test; (II) the medical history was incomplete; (III) the final diagnosis was unclear; and (IV) they were moribund and not expected to survive for 48 h due to irreversible primary diseases. Thirty lung cancer patients without any infectious diseases were enrolled in our study as the control group.
Diagnostic criteria for IPA
According to the standard criteria (11), the cases were classified into three categories: proven cases, probable cases and possible cases. A biopsy was needed for the proven cases, and histopathological evidence must be acquired. Microbiological evidence was demanded for the probable cases, and a host factor and a radiological criterion were also required. Microbiological proof was not demanded for the possible cases, although host factors and radiological criteria were needed. The colonization group was defined as patients with a positive Aspergillus culture from the sputum or BALF whose clinical symptoms were relieved without antifungal therapy.
BALF procedure and sample collection
A bronchoalveolar lavage (BAL) was performed, and samples (serum, plasma and BALF) were collected for measurement on the day when IPA was suspected. The guideline of the British Thoracic Society (BTS) was followed during the procedure (12). The segment selected for the BAL was guided by a recent chest CT scan, and the right middle lobe was selected when diffuse infiltrates were present. A 50 mL saline solution was instilled two times and aspirated gently. BALF was collected from the suction channel and stored in a sterile tube for microbiological culture, microscopic examination and further analysis, including GM and urea measurement.
GM and urea detection
Urea was detected in both of the plasma and BALF based on the following reaction principle: urea is hydrolysed by urease to generate ammonium carbonate, and then 2-ketoglutarate reacts with ammonium to produce L-glutamate in the presence of GLDH and NADH. In this reaction, 2 mol of NADH are oxidized to NAD+ when 1 mol of urea is hydrolysed. Thus, a decrease in the NADH concentration reflects the urea concentration, which is measured by spectrophotometric analysis.
The urea nitrogen assay was carried out on the Hitachi 7600 automatic biochemical analyser according to the manufacturer’s handbook. GM antigen was measured in both the sera and BALF with a double-sandwich enzyme-linked immunosorbent assay (ELISA) using the Platelia Aspergillus enzyme immunoassay (Bio-Rad, USA).
Each 300 µL BALF sample was used to determine the GM and urea levels. Positive and negative controls were included in each assay. The corrected GM concentration in the BALF sample was calculated as:
(Corrected BALF GM) = (BALF GM) × (Urea plasma)/(Urea BALF)
Data collection and clinical assessment
The demographic information and clinical characteristics of each patient enrolled in our study were obtained from the hospital’s electronic medical records, which included age, gender, comorbidities, body mass index (BMI), the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and the Sequential Organ Failure Assessment (SOFA) score. The chest CT scan, BALF culture and histological evidence were also recorded, as were the GM and urea concentrations.
Statistical analysis
Data from the study were analysed with SPSS 22.0 (IBM for Windows). Categorical variables were compared with the Chi-square test, and the results were presented as percentages (n). Continuous variables were reported as the means when the data were normally disturbed or as medians with 25 to 75 interquartile ranges for skewed data. Differences among means were analysed with the Kruskal-Wallis test to compare three or more groups.
According to the guidelines of IDSA and ESCMID, the proven and probable cases of IPA should be treated early to improve the prognosis while the possible cases could remain under observation (5,6). Therefore, the proven and probable cases of IPA were defined as the gold standard in our study and the receiver operating characteristic curve (ROC) analysis was performed to determine the optimal cut-off value. A two-sided P<0.05 was considered statistically significant. The ROC was generated using MedCalc 15.0, and the other figures were drawn with GraphPad Prism 7.0.
Results
Patient characteristics
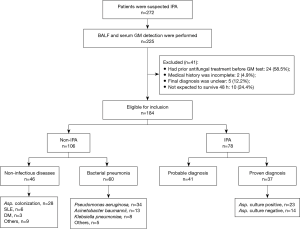
GM was detected in BALF and serum samples from 225 patients with suspected IPA during the study period, 41 (18.2%) of whom were excluded due to an unclear final diagnosis, moribund state and other reasons. Finally, 184 patients were enrolled in our study. Among them, 78 patients (42.4%) were diagnosed with IPA, and the other 106 patients were not diagnosed. IPA was proven in 37 patients from a lung biopsy, and 28 patients were diagnosed with Aspergillus colonization in the non-IPA group (Figure 1).
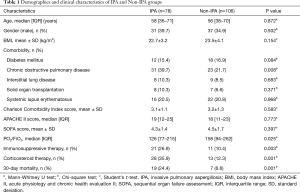
The clinical characteristics of the IPA and non-IPA groups are listed in Table 1. The age range, gender, BMI, and Charlson Comorbidity Index, APACHE II and SOFA scores did not significantly differ between the two groups (P>0.05). The proportions of patients with diabetes mellitus, interstitial lung disease, solid organ transplantation and systemic lupus erythematosus were also similar between the two groups (P>0.05), whereas more patients with chronic obstructive pulmonary disease (COPD) were detected in the IPA group (39.7% vs. 21.7%, P<0.01). The value of PO2/FiO2 in the IPA group [126 (IQR, 77–215)] was much lower than that in the non-IPA group [158 (IQR, 98–262)] (P<0.05). A higher percentage of patients who received immunosuppressive treatment (26.9% vs. 10.4%) and corticosteroid therapy (35.9% vs. 12.3%) was found in the IPA group than in the non-IPA group (P<0.05). The 30-day mortality rate was significantly higher in the IPA group and was almost 4-fold that in the non-IPA group (24.4% vs. 6.6%, P=0.001).
Full table
GM and (1→3)-β-D-glucan detection
The value of GM in serum samples was not found to be significantly different between the IPA proven group [1.02 ODI (IQR, 0.54–1.38)] and the IPA probable diagnosis group [1.04 ODI (IQR, 0.65–1.38)] (P>0.05). The serum GM concentration was significantly higher in the IPA cohort than in all of the non-IPA groups (P<0.001), (Figure 2A). The median uncorrected BALF GM value in the IPA proven cohort was 1.04 ODI (IQR, 0.86–1.45), which was higher than the values in all non-IPA groups (P<0.001) (Figure 2B).
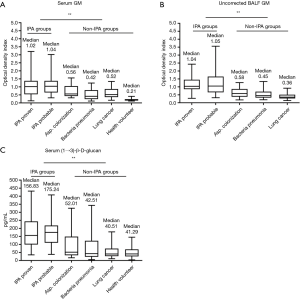
As a conventional biomarker for IPA discrimination, (1→3)-β-D-glucan (BDG) was also measured in all 184 included patients. The BDG values were not significantly different among the lung cancer control group [40.51 pg/mL (IQR, 27.33–74.25 pg/mL)], bacterial pneumonia group [42.51 pg/mL (IQR, 23.07–122.56 pg/mL)] and Aspergillus colonization group [52.01 pg/mL (IQR, 32.57–1.48.2)] (P>0.05). The BDG levels in the IPA group were higher than those in the non-IPA group (P<0.001) (Figure 2C).
GM and BDG were also detected in thirty serum samples from health volunteers. It indicated that the concentrations of GM [0.21 ODI (IQR, 0.15–0.23)] and BDG [41.29 pg/mL (IQR, 25.39–70.18 pg/mL)] were lower than those in IPA groups respectively (P<0.001).
Corrected BALF GM value and urea dilution
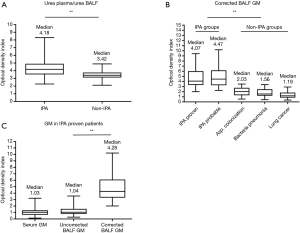
Figure 3A displays the urea plasma-to-urea BALF ratios. The median value of this ratio in the IPA group [4.18 (IQR, 3.52–4.91)] was greater than that in the non-IPA group [3.42 (IQR, 3.12–3.76)] (P<0.001). The corrected BALF GM values were not significantly different between the IPA proven diagnosis group and the IPA probable diagnosis group. However, the values in these two groups were approximately two times higher than that in the non-IPA group (P<0.001) (Figure 3B, Table 2).

Full table
The GM values in the 37 patients with a lung biopsy (IPA proven) were also compared (Figure 3C). The corrected BALF GM values [4.28 ODI (IQR, 3.30–6.12)] were considerably higher than the uncorrected values [1.04 ODI (IQR, 0.78–1.57)] (P<0.001). No difference was found between the GM values in the serum samples [1.03 ODI (IQR, 0.64–1.35)] and the uncorrected BALF samples (P>0.05).
Correlation of GM among different samples
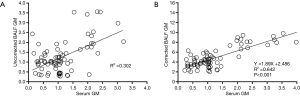
A scatter plot was applied to determine whether correlations existed between the GM values in the serum and BALF samples. No significant correlation was identified between GM in the uncorrected BALF and serum samples (R2 =0.302, P>0.05). However, when the GM values in the BALF sample were corrected by urea dilution, a significant correlation was detected (R2 =0.642, Y =1.89X +2.486, P<0.001) (Figure 4).
Diagnostic efficiency of GM for diagnosing IPA at different cut-off values
Because no consensus exists on the optimal cut-off value of GM in serum and BALF samples in the practice guidelines, meta-analysis or randomized controlled trials (5,6,13-15), the diagnostic efficiency of GM for IPA at different cut-off values was verified for our 184 enrolled patients. Lower accuracy (61.7%) was found in serum samples when the optimal cut-off value was defined as ≥0.5 ODI and ≥1.0 ODI (71.4%). In the uncorrected BALF samples, when the cut-off value was defined as a GM concentration ≥0.8 ODI, the accuracy was as high as 81.6%, with a better positive predictive value (PPV) (78%) and negative predictive value (NPV) (84%) than those of the other cut-off values (Table 3).

Full table
Diagnostic efficiency of different variables for diagnosing IPA
The ROC curves are shown in Figure 5. A cut-off value of 2.94 ODI for the corrected BALF GM resulted in a sensitivity of 85.91% and specificity of 94.07%, which corresponded to an area under the curve (AUC) of 0.961. A cut-off value of 0.86 ODI for the uncorrected BALF GM led to a low sensitivity of 74.36% and specificity of 88.14%. The AUC was 0.832, which was lower than that of the corrected BALF GM value (P<0.05). The optimal cut-off value for serum GM was 0.87 ODI, which led to an AUC of 0.746 and both lower sensitivity (64.11%) and specificity (80.51%) (Table 4).
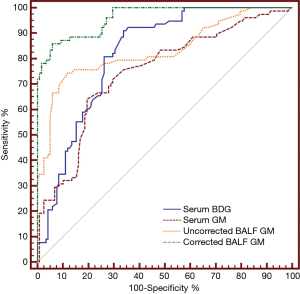
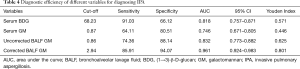
Full table
Diagnostic efficiency of the modified AspICU clinical algorithm
Thirty-seven patients diagnosed with IPA by lung biopsy were enrolled in our study. The Aspergillus culture results from sputum or BALF samples were negative in 14 (37.8%) patients, who would be misdiagnosed by application of the AspICU clinical algorithm. According to the optimal cut-off value for the corrected BALF GM (2.94 ODI), one criterion in the AspICU clinical algorithm (“Aspergillus-positive lower respiratory tract specimen culture”) was changed to “Aspergillus-positive lower respiratory tract specimen culture or a corrected BALF GM value ≥2.94 ODI”. High sensitivity and specificity (both 100%) were revealed with a satisfactory AUC (1.00), which was verified in the 37 patients with a proven IPA diagnosis. For the 106 non-IPA patients in our study (including 28 patients with Aspergillus colonization), the NPV of modified AspICU clinical algorithm was 87.4% while the rate of accuracy was 86.3% which were still satisfactory.
Discussion
Accurately diagnosing IPA among ICU patients is challenging, although molecular and conventional approaches have been improved over the past two decades. Recently, BALF GM detection has been strongly recommended in the practice guidelines of the IDSA due to its high sensitivity, especially for neutropenic patients (5). However, the optimal cut-off value is unidentified due to differences in BALF dilutions, patient selection and BAL procedures (16). Moreover, evidence for application of BALF GM in nonneutropenic patients is limited. The ratio of the urea plasma-to-urea BALF concentrations was previously applied as an index of ELF dilution. Thus, we hypothesized that the BALF GM value corrected by urea dilution might serve as an adjunct for diagnosis of IPA among nonneutropenic patients.
As indicated in our study, the proportion of patients with COPD was higher in the IPA group than in the non-IPA group (39.7% vs. 21.7%, P<0.01). Possible explanations could be alterations of the alveolar structure, decreased mucociliary clearance, mucosal lesions and an impaired immunologic response, which might lead to frequent hospital stays, invasive procedures, such as intubation, and a long course of antibiotics treatment (17). The other two risk factors were immunosuppressive use (26.9% vs. 10.4%, P<0.01) and corticosteroid therapy (35.9% vs. 12.3%, P<0.01) due to the accompanying humoral and cellular immunity functional disorders (18). Not unexpectedly, a dismal prognosis of IPA was also found in our study, which led to a long-term search for better therapeutic strategies.
Generally, the optimal cut-off value for GM in sera is set at 0.5 ODI, but many factors can lead to a false-positive or false-negative result (1). As shown in our study, the serum GM value in the lung cancer group was 0.52 ODI (IQR, 0.36–0.87), which could be interpreted as albumin treatment in cancer patients. We also found that the serum GM value was greater than 0.5 ODI in 26 (43.3%) patients with bacterial pneumonia due to the prescription of piperacillin-tazobactam for Pseudomonas aeruginosa treatment. Thus, the PPV was only 51% and the NPV was 81% when the cut-off value was defined as 0.5 ODI for serum GM in our study.
Although GM in BALF samples was reported to have better sensitivity than that in sera (19), the optimal cut-off value varied significantly. We measured the diagnostic efficiency of different cut-off values of uncorrected BALF GM (0.5, 0.8 1.0, 1.5, 2.0 and 3.0) reported by previous clinical trials. An uncorrected BALF GM value of 0.8 ODI might be the best choice with 81.6% diagnostic accuracy, although the sensitivity (75.6%) and PPV (78%) were still unsatisfactory.
To overcome the problem, BALF was corrected by urea dilution in our study. The urea plasma-to-urea BALF ratios in the IPA group was higher than that in the non-IPA group. The value of PO2/FiO2 in the IPA group was much lower than that in the non-IPA group which indicated that more patients were found under oxygen deficit circumstances in the IPA group and resulted in poor tolerance to BAL. More coughing and shortness of breath occurred during the procedure, and less urea was obtained from the BALF samples in the IPA group.
IPA is primarily caused by inhalation of fungal spores, and BALF samples are taken from the site of infection. Thus, the GM concentration in the BALF sample can be reasonably expected to be higher than that in the serum (7,20,21). The BALF GM level was corrected by urea dilution in our study, and we found that the corrected BALF GM values were more than quadruple the uncorrected values in the IPA-proven patients.
Non-corrected BALF GM values could be altered due to the different volumes obtained by BAL, and that the urea dilution method could standardise these figures. The ROC curve analysis verified that a 2.94 ODI for the corrected BALF GM concentration was an optimal cut-off value, with sensitivity as high as 85.91% and 94.07% specificity. The AUC was 0.961, which was a satisfactory index. Compared to that of the uncorrected BALF GM values, the corrected values showed much higher sensitivity and specificity, which might decrease the rate of misdiagnosis or missed diagnosis.
A lack of correlation between the BALF and serum GM values was revealed among the IPA patients in our study. This lack might be interpreted as meaning that the BALF dilution was diverse and uncontrollable, which concurred with the studies of Duettmann et al. and Jackson et al. (21,22). Therefore, we attempted to minimize the confounding factors and corrected the BALF GM value by urea dilution, which showed a statistically significant correlation between the corrected BALF GM value and the serum GM value (R2 =0.642, P<0.001).
The isolation rate of Aspergillus spp. was only 50% in the respiratory samples from the IPA patients, which was far from satisfactory (3). In addition, positive culture results always raise a clinical dilemma, because ICU doctors must discriminate colonization from real infection. Moreover, a negative culture result cannot rule out infectious diseases. Based on the theories proposed above and the optimal corrected BALF GM value (2.94 ODI), one criterion in the AspICU clinical algorithm (“Aspergillus-positive lower respiratory tract specimen culture”) was changed to “Aspergillus-positive lower respiratory tract specimen culture or a corrected BALF GM value ≥2.94 ODI”. The modified algorithm was verified using the 37 patients with an IPA diagnosis based on histopathological evidence, and high sensitivity and specificity (both 100%) were revealed with a satisfactory AUC (1.00). These findings indicated that the modified AspICU clinical algorithm might be applied as a reliable diagnostic instrument in clinical settings in the future.
To the best of our knowledge, this study is the first prospective study to correct the BALF GM value by urea dilution and modify the AspICU clinical algorithm accordingly. However, some limitations still exist. First, the number of patients who underwent biopsy was limited (n=37). Although histopathological evidence was recommended as a gold standard in the IDSA guideline (5), biopsy was uncommon in ICU admission patients because of the high incidence of complications, such as bleeding or pneumothorax. Second, we were unable to recruit health volunteers as a control group because BAL in these samples was disapproved by the Ethics Committee. Only blood samples from health volunteers were obtained from our study to determine the baseline value of BDG and GM. Lastly, some cases of false-positive BALF GM were unavoidably enrolled in our study, which led to unnecessary antifungal treatment. The incidence was reported to be as high as 20% (23,24). Further studies investigating new ideal marker verification and methods to discriminate false-positive results are needed in the future.
In summary, we demonstrated the potential of corrected BALF GM values by urea dilution as a biomarker for the diagnosis of IPA with high accuracy. The modified AspICU clinical algorithm based on the corrected BALF GM value should become a reliable diagnostic instrument in clinical settings.
Supplementary file 1
AspICU criteria
Putative invasive pulmonary aspergillosis (all four criteria must be met)
- Aspergillus-positive lower respiratory tract specimen culture;
- Compatible signs and symptoms (one of the following):
- Fever refractory to at least 3 days of appropriate antibiotic therapy;
- Recrudescent fever after a period of defervescence of at least 48 h while still on antibiotics and without other apparent cause;
- Pleuritic chest pain;
- Pleuritic rub;
- Dyspnoea;
- Haemoptysis;
- Worsening respiratory insufficiency despite appropriate antibiotic therapy and ventilator support.
- Abnormal medical imaging by portable chest X-ray or CT scan of the lungs;
- Either IVa or IVb:
- (IVa) Host risk factors (one of the following conditions);
- Neutropenia (absolute neutrophil count 500/mm3) preceding or at the time of ICU admission;
- Underlying haematological or oncological malignancy treated with cytotoxic agents;
- Glucocorticoid treatment (prednisone equivalent 20 mg/day);
- Congenital or acquired immunodeficiency.
- (IVb) Semi-quantitative Aspergillus-positive culture of BAL fluid without bacterial growth together with a positive cytological smear showing branching hyphae.
Acknowledgements
Funding: This work was supported by the National Key Research and Development Program of China (No. 2017YFC0909002), Scientific Research Project of Shanghai Municipal Health Bureau (No. 201840006), National Science Foundation of China (No. 81601745) and Key Discipline Construction Project of Pudong Health Bureau of Shanghai (grant No. PWZxk201709).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Shanghai Jiao Tong University (No. 2016-Clinical-Res-083), and informed written consent was obtained from either the patients or their next of kin.
References
- Paiva JA, Mergulhão P, Pereira JM. Aspergillus and other respiratory fungal infections in the ICU: diagnosis and management. Curr Opin Infect Dis 2018;31:187-93. [PubMed]
- Olaechea Astigarraga PM, Alvarez Lerma F, Zaldíbar Enriquez E. Invasive pulmonary aspergillosis in the non-neutropenic critical patient: future challenges. Med Intensiva 2006;30:386-91. [Crossref] [PubMed]
- Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest 2007;131:261-74. [Crossref] [PubMed]
- Blot SI, Taccone FS, Van den Abeele AM, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 2012;186:56-64. [Crossref] [PubMed]
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63:e1-60. [Crossref] [PubMed]
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 2018;24 Suppl 1:e1-38. [Crossref] [PubMed]
- Rennard SI, Basset G, Lecossier D, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986;60:532-8. [Crossref] [PubMed]
- Baughman RP. Technical aspects of bronchoalveolar lavage: recommendations for a standard procedure. Semin Respir Crit Care Med 2007;28:475-85. [Crossref] [PubMed]
- Ni W, Yang D, Mei H, et al. Penetration of Ciprofloxacin and Amikacin into the Alveolar Epithelial Lining Fluid of Rats with Pulmonary Fibrosis. Antimicrob Agents Chemother 2017;61. [Crossref] [PubMed]
- Andersen CU, Sonderskov LD, Bendstrup E, et al. Voriconazole Concentrations in Plasma and Epithelial Lining Fluid after Inhalation and Oral Treatment. Basic Clin Pharmacol Toxicol 2017;121:430-4. [Crossref] [PubMed]
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66 Suppl 3:iii1-21. [PubMed]
- He H, Ding L, Sun B, et al. Role of galactomannan determinations in bronchoalveolar lavage fluid samples from critically ill patients with chronic obstructive pulmonary disease for the diagnosis of invasive pulmonary aspergillosis: a prospective study. Critical care 2012;16:R138. [Crossref] [PubMed]
- Zou M, Tang L, Zhao S, et al. Systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLoS One 2012;7:e43347. [Crossref] [PubMed]
- Zhou W, Li H, Zhang Y, et al. Diagnostic Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary Aspergillosis. J Clin Microbiol 2017;55:2153-61. [Crossref] [PubMed]
- Yu Y, Zhu C, Gao Y. Bronchoalveolar lavage fluid galactomannan as a diagnostic biomarker for IPA: still a long way to go. Crit Care 2016;20:280. [Crossref] [PubMed]
- Delsuc C, Cottereau A, Frealle E, et al. Putative invasive pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary disease: a matched cohort study. Crit Care 2015;19:421. [Crossref] [PubMed]
- Lee C, Klaustermeyer WB. Effect of high dose inhaled corticosteroids on cell mediated immunity in patients with asthma. Allergol Immunopathol (Madr) 2012;40:100-3. [Crossref] [PubMed]
- Hope WW, Petraitis V, Petraitiene R, et al. The initial 96 hours of invasive pulmonary aspergillosis: histopathology, comparative kinetics of galactomannan and (1->3) beta-d-glucan and consequences of delayed antifungal therapy. Antimicrob Agents Chemother 2010;54:4879-86. [Crossref] [PubMed]
- Yu Y, Zhu C, Liu C, et al. Amphotericin B nebulisation for invasive pulmonary aspergillosis prophylaxis: the conflict of ideality and reality. Int J Antimicrob Agents 2017;49:263-4. [Crossref] [PubMed]
- Duettmann W, Koidl C, Troppan K, et al. Serum and urine galactomannan testing for screening in patients with hematological malignancies. Med Mycol 2014;52:647-52. [Crossref] [PubMed]
- Jackson AS, Sandrini A, Campbell C, et al. Comparison of biomarkers in exhaled breath condensate and bronchoalveolar lavage. Am J Respir Crit Care Med 2007;175:222-7. [Crossref] [PubMed]
- Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002;121:1988-99. [Crossref] [PubMed]
- Iversen M, Burton CM, Vand S, et al. Aspergillus infection in lung transplant patients: incidence and prognosis. Eur J Clin Microbiol Infect Dis 2007;26:879-86. [Crossref] [PubMed]


