
The clinical value of a new method of functional lymph node dissection in video-assisted thoracic surgery right non-small cell lung cancer radical resection
Introduction
Lung cancer is the most common cause of death for all cancers in the world and has the highest incidence among malignant tumors (1), with non-small cell lung cancer (NSCLC) accounting for approximately 80% (2). The eighth edition of the National Comprehensive Cancer Network guidelines clearly noted that surgical treatment remained the first choice for stages I and II, and for partial resection of stage III NSCLC, as an important method for curing lung cancer (3-5). Moreover, mediastinal lymph node metastasis was an important prognostic factor in patients with NSCLC; therefore, anatomical lobectomy combined with systematic lymph node dissection was thought to be the standard operative method for lung cancer radical resection (5-7), not only to avoid residual cancer and reduce tumor recurrence but also to ensure postoperative pathological TNM stage and to guide postoperative adjuvant therapy, which has important significance for improving patient survival (7-9).
However, in clinical practice, we found that there remained some deficiencies in routine systemic lymph node dissection, including large wounds created by lymph node dissection, much oozing of blood from the wound, poor protection of vagus nerve and its pulmonary branches, and a large volume of postoperative thoracic drainage. Based on the concept of minimally invasive surgery, precise surgery, and rapid recovery, our team first put forward a new method of lymph node dissection: tunnel-type en bloc mediastinal lymph node dissection. This dissection, similar to a mining tunnel, functionally dissects the lymph nodes of stations 2R/4R/7 and preserves the mediastinal pleura. This method not only ensures systematic and complete mediastinal lymph node dissection but also preserves the vagus nerve and its pulmonary branches, which is significant for effectively reducing postoperative pulmonary infection, chronic cough and other complications, to accelerate postoperative rehabilitation in patients with lung cancer.
According to the method of lymph node dissection of stations 2R/4R/7, patients were divided into the tunnel-type group and the routine group, and a retrospective comparative study was carried out to explore the clinical value of tunnel-type en bloc mediastinal lymph node dissection in video-assisted thoracic surgery (VATS) right NSCLC radical resection.
Methods
Patient selection
In this study, we retrospectively analyzed the clinical data of 196 patients who were diagnosed with right NSCLC in our hospital and underwent VATS right lung cancer radical resection from 1 Jan. 2013 to 31 Dec. 2015.
The inclusion criteria for patients were determined as follows: (I) VATS right anatomic lobectomy (pulmonary lobectomy/segmentectomy/sleeve lobectomy) and systemic lymph node dissection (including at least 3 groups of mediastinal lymph nodes); (II) no distant metastasis in preoperative examination; (III) stages I and II and partial resection of stage III NSCLC [Union for International Cancer Control (UICC) TNM classification, eighth edition]; (IV) no prior malignancy; (V) tolerance of general anesthesia; and (VI) there was no perioperative comprehensive treatment.
The exclusion criteria for patients were as follows: (I) conversion to thoracotomy or VATS with mini-incision from VATS; (II) intraoperative exploration revealed extensive pleural adhesions; and (III) previous right thorax operation.
All patients received routine preoperative examinations, which included chest CT scan, color Doppler of abdomen, fiberoptic bronchoscopy, cranial MRI, and bone imaging and cardiopulmonary function tests. There was no PET/CT in all preoperative examination because of lack of money. Overall, 101 patients were male, and 95 patients were female.
According to the method of lymph node dissection of stations 2R/4R/7, the patients were divided into two groups. Patients with tunnel-type en bloc mediastinal lymph node dissection were designated as the tunnel-type group (n=102), and those with routine mediastinal lymph node dissection were the routine group (n=94), undergoing surgery before 1 Jul. 2014. The general clinical data of the two groups are displayed in Table 1. There was no significant difference between the groups (P>0.05), and the baseline data of the patients were comparable. This study has been approved by the Hospital Ethics Committee (the number: 2018KY033).
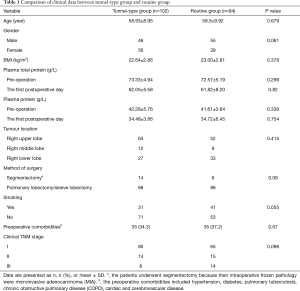
Full table
Surgical approaches
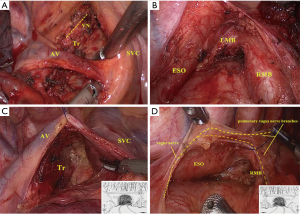
All patients had been informed of the method of right lung cancer radical resection and signed the operative informed consent. Both groups first underwent VATS anatomic lobectomy (pulmonary lobectomy/segmentectomy/sleeve lobectomy), then completed systematic lymph node dissection. Systematic lymph node dissection was required to ensure the standard extent and integrity of not fewer than 3 stations of station N1 and 3 stations of station N2, including subcarinal lymph nodes; the lymph nodes were completely removed with the surrounding adipose tissue in each group (10-12). Regardless of tunnel-type or routine systematic lymph node dissection, the Martini method was used for the extent of lymph node dissection (13). The regional lymph node dissection was as follows: intrapulmonary lymph nodes (station 11th–13th), hilar lymph nodes (station 10th), lower mediastinal lymph nodes (station 8th–9th), subcarinal lymph node (station 7th), upper mediastinal lymph nodes (station 2nd and 4th). The operative processes are described below (Figure 1).

Tunnel-type en bloc mediastinal lymph node dissection
Lymph node dissection of station 7
After the posterior mediastinum was exposed, the mediastinal pleura was opened transversely with the electric hook from the upper margin of the inferior pulmonary vein to the left side in the front of the vagus nerve, then to the right side in the right main bronchus, approximately 4–5 cm. The pulmonary vagus nerve branches were not disconnected. The assistant lifted the mediastinal pleura with the endoscopic gastric forceps, and the surgeon continued to free the loose connective tissue below the mediastinal pleura of station 7 area and suspended the mediastinal pleura with 3-0 proline to fully expose the surgical visual field and operative space. Then, the assistant dragged the subcarinal lymph nodes with the endoscopic gastric forceps. The surgeon spirally dissociated the lymph nodes with an ultrasound knife, starting from the front of the esophagus and following the posterior pericardium and the lateral wall of the right main bronchus to the lower edge of the bronchus, which was removed completely with its surrounding adipose tissue, as if digging a tunnel. It is important to avoid damaging the vagus nerve pulmonary branches (Figure 2).
Lymph node dissection of station 2R/4R
After the superior mediastinum was exposed, the surgeon freed the fibrous connective tissue close to the arch of the azygos vein with an ultrasound knife and did not open the mediastinal pleura overlying the level of the 2R/4R area. The arch of the azygos vein was suspended with 3-0 proline to fully expose the surgical visual field and operative space. Then, the assistant dragged the subcarinal lymph nodes with the endoscopic gastric forceps. The surgeon spirally dissociated the lymph nodes with an ultrasound knife, starting from the lateral wall of the trachea, following the posterior margin of the superior vena cava, until the lymph nodes with their surrounding adipose tissue were completed removed, as if digging a tunnel. The procedure not only preserved the superior mediastinal pleura but also reduced stimulation of the vagus nerve (Figure 2).
Outcome measures
The outcome measures were as follows: age, gender, body mass index, plasma total protein, plasma protein, tumor location, method of anatomic lobectomy, smoking, and preoperative comorbidities.
The indexes of safety and feasibility were operative time, time of lymph node dissection of station N2, amount of bleeding, postoperative thoracic drainage volume, extubation time (there was no leak in the bottle when coughing, and the chest tube was pulled when the drainage volume was less than 200 mL), hospitalization days, postoperative pulmonary infection, postoperative pathological data, number of mediastinal lymph node dissection, and postoperative chronic cough (cough has lasted six months, alongside loss of appetite, pain, and shortness of breath, was a significant predictor of quality of life).
The prognostic indexes for survival were 3-year recurrence and metastasis and 3-year survival.
Postoperative follow-up data
All patients were followed up by telephone and out-patient review, carried out once every three months for the first 2 years and once every 6 months thereafter. The follow-up project included whether the patient had a chronic cough, the recurrence and metastasis time, and the survival time. The survival time was calculated from the date of the operation, and the deadline of follow-up was scheduled for 30 Sep. 2017 or the date of the death from lung cancer or loss to follow-up. Postoperative chronic cough was defined as coughing that lasted more than three months after lung cancer surgery.
Statistical analyses
Statistical analyses were performed on SPSS software, version 19.0. The α (error probability) was set to 0.05. The measurement data were expressed by mean ± SD, and the mean value was compared with the normality test and homogeneity test of variance. The t-test was used after the data fulfilled the criteria of normal distribution and equal variance; otherwise, the Wilcoxon rank sum test was used. The count data were analyzed by the χ2 test or Fisher’s exact test. The survival rate, recurrence and metastasis rate of all patients were calculated using Kaplan-Meier plots (log-rank test).
Results
Perioperative index and postoperative complications
All patients underwent VATS right anatomic lobectomy and systematic lymph node dissection. There were no deaths or intensive care unit treatments during the perioperative period. There were statistically significant differences between the tunnel-type group and the routine group, including the amount of bleeding, postoperative thoracic drainage volume, and postoperative extubation time. However, for the time of lymph node dissection of station N2 and operative time, there were no significant differences between the groups (P>0.05).
The incidence of postoperative complications was 8.8% vs. 17.0% for the tunnel-type group and the routine group, respectively; these complications included postoperative pulmonary infection in 2 cases, pulmonary air leak in 5 cases, chylothorax in 2 cases, atrial fibrillation (AF) in 1 case in the tunnel-type group as well as pulmonary infection in 9 cases, pulmonary air leak in 2 cases, chylothorax in 4 cases, and AF in 6 cases in the routine group. In the routine group, there was 1 patient who underwent a second operation for thoracic hemostasis. Pulmonary infection in the two groups showed a significant difference (P<0.05).
There was no significant difference in terms of the size and pathological type of the tumor, visceral pleura involvement, pTNM stage, and T/N stage between the groups (P>0.05). The primary tumor of patients with T4 stage at postoperative pathology was located in a different right lobe. The patients with perioperative indexes and postoperative complications are shown in Table 2.
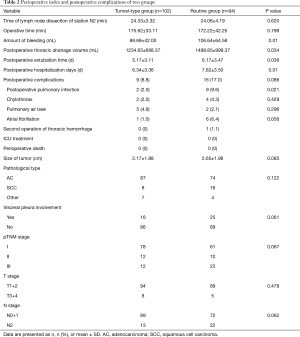
Full table
Mediastinal lymph node dissection of station 2R/4R/7
There was no significant difference in terms of total number, positive number and metastasis rate of lymph node dissection of stations 2R/4R/7 between the groups (P>0.05), which proved that the result was equivalent between the tunnel-type en bloc and routine lymph node dissection. Mediastinal lymph node dissections of patients are shown in Table 3.

Full table
All patients were followed up to determine whether they had a chronic cough. Compared to the routine group (66/94, 70.21%), there was less chronic cough in the tunnel-type group (55/102, 53.92%), with a significant difference between the groups (P=0.019).
Prognostic evaluation of the tunnel-type group
Recurrence and metastasis and survival of all patients
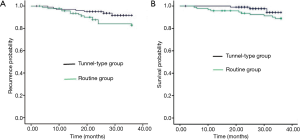
All 196 patients were reviewed and followed up, and 11 cases were lost, including 5 cases in the tunnel-type group and 6 cases in the routine group. The median survival for the routine group was 28 months [95% confidence interval (CI), 13–38 months], and the median survival for the tunnel-type group was 43 months (95% CI, 5–56 months). There was no significant difference in 3-year recurrence and metastasis by log-rank test between the groups (8.3% vs. 17.1%, P=0.083) (Figure 3A). There were 7 patients in the tunnel-type group, including 2 cases of local recurrence, 4 cases of distant metastasis, and 1 case of local recurrence with distant metastasis. There were 15 patients in the routine group, including 3 cases of local recurrence, 9 cases of distant metastasis, and 3 cases of local recurrence with distant metastasis. The 3-year survival showed no significant difference by log-rank test (94.3% vs. 88.9%, P=0.146) (Figure 3B).
Discussion
At present, although the combined modality therapy of NSCLC is increasingly mature and perfect, lung cancer radical resection remains the first choice of treatment for the resectable NSCLC. Lung cancer is a disease that is prone to mediastinal lymph node metastasis, reported to occur in 30–40% of cases (15), and the presence of lymph node micrometastasis has been reported (16,17). Many scholars believe that systemic lymph node dissection plays an important role in postoperative prognosis of lung cancer. However, in clinical practice, we found that routine systemic lymph node dissection had some deficiencies, including large wounds from lymph node dissection, as well as poor protection of the vagus nerve and its pulmonary branches. Therefore, it is important to functionally and completely dissect mediastinal lymph nodes while protecting the vagus nerve and its pulmonary branches.
Based on the principle of continuous pursuit of minimally invasive surgery, precise surgery, and rapid recovery, our team remains committed to more minimally invasive surgical treatment; therefore, we first proposed the new method of functional lymph node dissection: tunnel-type en bloc mediastinal lymph node dissection. The surgical method was spiraling, bloodless and hierarchical to remove mediastinal lymph nodes as if digging a tunnel, functionally dissecting the lymph nodes of stations 2R/4R/7 and optimizing the deficiencies of routine lymph node dissection. This method can not only ensure systematic and complete mediastinal lymph node dissection but also preserve the vagus nerve and its pulmonary branches as well as the mediastinal pleura, reducing the exudation and bleeding of the wound with the packing of hemostatic material.
The vagus nerve is widely distributed in the lung, forming pulmonary vagus nerve branches. Many physiological functions of the lung are regulated by the vagus nerve; its stimulation causes bronchoconstriction, mucus secretion and bronchovascular vasodilation. The most important factor is that pulmonary vagus nerve branches innervate the pulmonary cough and stretch reflexes, preventing pulmonary hyperventilation (18,19). In addition, the vagus nerve regulates inflammatory responses by mechanical or chemical reactions and nutrition. Therefore, the protection of the pulmonary vagus nerve branches is the core of mediastinal lymph node dissection in lung cancer radical resection.
Compared with the routine group, the amount of bleeding in the tunnel-type group was significantly lower (P<0.05). The results showed that the tunnel-type en bloc mediastinal lymph node dissection did not prolong the operative time (24.33±3.32 vs. 24.06±4.19 min, P>0.05), but the amount of bleeding was reduced (89.69±42.00 vs. 106.64±64.58 mL, P<0.05); additionally, there were no second operations for postoperative pleural hemorrhage. This finding highlighted the many advantages of the bloodless and hierarchical dissection of the tunnel-type group, including that the anatomical level was more obvious and the lymphatic vessels were revealed more clearly, effectively reducing the risk of intraoperative and postoperative bleeding. However, because the operative space of the tunnel-type en bloc mediastinal lymph node dissection was smaller, the surgeon and the assistant had a higher demand for thoracoscopic surgery skills and mastery of the anatomical structures.
The incidence of pulmonary complications in the tunnel-type group was lower (2% vs. 9.6%, P<0.05). In many studies, it was reported that the pulmonary vagus nerve branches play an important role in the cough reflex, sputum excretion and immune defense of pulmonary inflammation after surgery (20-22). Therefore, we believed that the protection of the vagus nerve and its pulmonary branches in the tunnel-type en bloc mediastinal lymph node dissection was of great significance to reduce postoperative pulmonary infection.
In addition, arrhythmia was one of the common postoperative complications of lung cancer, possibly related to factors such as postoperative incision pain, hypoxia, and injury to the vagus nerve and its cardiac branches. With both groups undergoing the same treatment and patients in neither group having a history of arrhythmia or coronary heart disease before surgery, the results showed that the incidence of AF in the tunnel-type group was lower than that of the routine group. According to the clinical and experimental study of Schauerte et al. (23), that proved that the vagus nerve played an important role in the induction and maintenance of AF, we believed that it had an important influence on the decrease in postoperative arrhythmia to reduce the injury of the vagus nerve in the tunnel-type en bloc mediastinal lymph node dissection. However, there was no significant difference in the incidence of postoperative AF between the groups (P>0.05), possibly related to the small sample size of this study. Postoperative thoracic drainage volume, extubation time and hospitalization days in the tunnel-type group were reduced compared to those of the routine group (P<0.05). The results of this study showed that the preservation of the mediastinal pleura and the packing of hemostatic materials had significant advantages toward protecting and compressing the operative wound, possibly reducing postoperative lymphatic exudation. Tunnel-type en bloc mediastinal lymph node dissection could reduce postoperative thoracic drainage volume, accelerate extubation, shorten hospitalization days, and accelerate postoperative rehabilitation. Therefore, we believe that VATS tunnel-type en bloc mediastinal lymph node dissection is safe and feasible, agreeing with the concept of rapid surgical rehabilitation.
The tunnel-type en bloc mediastinal lymph node dissection could functionally dissect the lymph nodes of stations 2R/4R/7 and strongly protect the vagus nerve and its pulmonary branches, effectively reducing the incidence of postoperative infection. Therefore, we wondered whether it was possible to reduce the incidence of postoperative chronic cough. To this end, we followed up the chronic cough of patients after surgery. Compared with the routine group, the tunnel-type group had less postoperative chronic cough. There was a significant difference between the groups (P<0.05), which proved that the functional protection of the vagus nerve and its pulmonary branches effectively reduce the incidence of postoperative chronic cough and improve the quality of the patients’ lives.
The results of the study showed that, by the thoroughness of lymph node dissection of stations 2R/4R/7, the tunnel-type en bloc mediastinal lymph node dissection was not inferior to routine mediastinal lymph node dissection. There were no significant differences between the groups in terms of total number of lymph nodes of station 2R/4R (7.66±4.54 vs. 8.90±4.55, for the tunnel-type group and the routine group, respectively, P=0.057) or in the positive number of lymph nodes of station 2R/4R (0.16±0.73 vs. 0.40±1.14, for the tunnel-type group and the routine group, respectively, P=0.075). For subcarinal lymph node dissection, the total number and positive number were not significantly different between the groups (P>0.05). There were no significant differences in lymph node dissection time and total number of lymph nodes of station N2 between the groups (P>0.05).
In the NSCLC radical resection, we insisted on carrying out the principle of radical resection. The results showed that there were no significant differences between the groups in terms of total number, positive number and incidence of metastasis of lymph nodes of stations 2R/4R/7, excluding an effect of the difference in the number or station of lymph node dissection on postoperative survival of the patients.
There remained a question as to whether the method of tunnel-type en bloc mediastinal lymph node dissection, which maintained the mediastinal pleura, affected postoperative prognosis. Through further research and analysis, we found that the difference in 3-year recurrence and metastasis between the groups was not significant (P=0.083), but the recurrence in the tunnel-type group was lower than that in the routine group (8.3% vs. 17.1%). There was no significant difference in 3-year survival between the groups (P=0.146): the tunnel-type group was 94.3%, and the routine group was 88.9%. As shown in the survival curve, the survival statistics of the two groups gradually separated, and the curve for the tunnel-type group was higher.
There were some limitations in this study, including the following: (I) the patients in the two groups were not operated on during the same period; this was a retrospective study, and there was some bias although the two groups had a good comparability; (II) the sample size of the groups was small, and a prospective randomized controlled study should be performed at a later stage; (III) the postoperative chronic cough in both groups lacked objective evaluation indexes, such as the frequency of cough or the effect on quality of life, which needs to be followed up; and (IV) the median follow-up time of the tunnel-type group was shorter (28 months). If the long-term curative effect of the two surgical methods are accurate, the follow-up time should be extended further.
Conclusions
Compared with routine mediastinal lymph node dissection, tunnel-type en bloc mediastinal lymph node dissection had similar quality and standard mediastinal lymphadenectomy of station N2. Moreover, it had more advantages: (I) the new surgical method protected the vagus nerve and its pulmonary branches, effectively reducing postoperative pulmonary infection and chronic cough; and (II) it preserved the mediastinal pleura, reducing postoperative exudation, accelerating postoperative extubation, and shortening hospitalization days. However, the method did not increase postoperative recurrence and metastasis, and it did not reduce survival of patients. Therefore, we believe that the tunnel-type en bloc mediastinal lymph node dissection is a safe, thorough and feasible surgical method, worthy of being popularized and applied for VATS right NSCLC radical resection.
Acknowledgements
Funding: This study was supported by Fujian Young Teacher Fund (JAT160209), Startup Fund for scientific research, Fujian Medical University (2016QH036), National Natural Science Foundation of China (Grant No. 81773129), Fujian Provincial Natural Science Foundation (2017J01291), Fujian Medical Innovation Fund (2014-CX-15), Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant number: 2017Y9013).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study has been approved by the Hospital Ethics Committee (the number: 2018KY033).
References
- Torre LA, Bray F, L, Siegel R, et al. Global cancer statistics 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Darling GE, Maziak DE, Inculet RI, et al. Positron emission tomography-computed tomography compared with invasive mediastinal staging in non-small cell lung cancer: results of mediastinal staging in the early lung positron emission tomography trial. J Thorac Oncol 2011;6:1367-72. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; Discussion 762-4. [Crossref] [PubMed]
- Kates M, Swanson S, Wisnivesky JP. Survival following lobectomy and limited resection for the treatment of stage I non-small cell lung cancer<=1 cm in size: a review of SEER data. Chest 2011;139:491-6. [Crossref] [PubMed]
- Yano M, Yoshida J, Koike T, et al. Survival of 1737 lobectomy-tolerable patients who underwent limited resection for cStage IA non-small-cell lung cance. Eur J Cardiothorac Surg 2015;47:135-42. [Crossref] [PubMed]
- Deslauriers J. Mediastinal lymph nodes: ignore? sample? dissect? The role of mediastinal node dissection in the surgical management of primary lung cancer. Gen Thorac Cardiovasc Surg 2012;60:724-34. [Crossref] [PubMed]
- Hytych V, Taskova A, Horazdovsky P, et al. Importance of systemic mediastinal lymphadenectomy in exact staging of bronchogenic carcinoma. Bratisl Lek Listy 2013;114:569-72. [PubMed]
- Watanabe S, Asamura H. Lymph node dissection for lung cancer: significance, strategy, and technique. J Thorac Oncol 2009;4:652-7. [Crossref] [PubMed]
- Verhagen AF, Bulten J, Shirango H, et al. The clinical value of lymphatic micrometastases in patients with non-small cell lung cancer. J Thorac Oncol 2010;5:1201-5. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. [Crossref] [PubMed]
- Shapiro M, Kadakia S, Lim J, et al. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest 2013;144:1615-21. [Crossref] [PubMed]
- Martini N. Mediastinal lymph node dissection for lung cancer. The Memorial experience. Chest Surg Clin N Am 1995;5:189-203. [PubMed]
- Chen S, Huang S, Yu S, et al. Tunnel-type en bloc mediastinal lymph node dissection in video-assisted thoracic surgery right non-small cell lung cancer radical resection. Asvide 2019;6:051. Available online: http://www.asvide.com/article/view/30110
- Ding N, Mao Y. Advances in Lymph Node Metastasis and the Modes of Lymph Node Dissection in Early Stage Non-small Cell Lung Caner. Zhongguo Fei Ai Za Zhi 2016;19:359-63. [PubMed]
- Li J, Li ZN, Yu LC, et al. Gene diagnosis of micrometastases in regional lymph nodes of patients with stage I non-small cell lung cancer: impact on staging and prognosis. Clin Transl Oncol 2013;15:882-8. [Crossref] [PubMed]
- Dai CH, Li J. Molecular diagnosis and prognostic significance of lymph node micrometastasis in patients with histologically node-negative non-small cell lung cancer. Tumour Biol 2013;34:1245-53. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Luyer MD, et al. Topography and extent of pulmonary vagus nerve supply with respect to transthoracic oesophagectomy. J Anat 2015;227:431-9. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Luyer MD, et al. Preserving the pulmonary vagus nerve branches during thoracoscopic esophagectomy. Surg Endosc 2016;30:3816-22. [Crossref] [PubMed]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009;9:418-28. [Crossref] [PubMed]
- Belvisi MG. Overview of the innervation of the lung. Curr Opin Pharmacol 2002;2:211-5. [Crossref] [PubMed]
- Mazzone SB, Canning BJ. Autonomic neural control of the airways. Handb Clin Neurol 2013;117:215-28. [Crossref] [PubMed]
- Schauerte P, Scherlag BJ, Patterson E, et al. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol 2001;12:592-9. [Crossref] [PubMed]


