Vaspin and lipocalin-2 levels in severe obsructive sleep apnea
Background
Obstructive sleep apnea syndrome (OSAS) is a disorder characterized by repetitive obstructions of the upper airway with a prevalance of 2-4% (1-3). Repetitive pauses of airflow cause a reduction in oxygen saturation (4). OSAS is usually associated with obesity, increased cardiovascular disease, hypertension, dyslipidemia and diabetes mellitus (5-7).
Adipocytes secrete numerous molecules called adipocytokines which are suspected to have a role in the pathogenesis of metabolic syndrome (MS) (8). Vaspin and lipocalin-2 are the two members of this adipocytokine family. A serine protease inhibitor, vaspin is an insulin-sensitizing adipocytokine. An increase in serum vaspin levels was suggested to be a compensatory response to antagonize the activity of the proteases expressed in insulin resistance and obesity. In another words, a high vaspin level has a defensive effect against insulin resistance (9).
Lipocalin-2 was reported to be associated with obesity and insulin resistance in humans and rats (10). Similarly, an association between lipocalin-2 and MS, dyslipidemia, hyperinsulinemia and hyperglycemia was also reported. Lipocalin-2 levels were found higher in patients with coronary heart disease (11).
Studies on vaspin and lipocalin-2 pointed out the importance of these molecules for a better understanding of MS and its components. Recently, Kim et al. suggested that the presence of OSAS even in nonobese individuals was significantly associated with impaired glucose metabolism, which can be responsible for future risk for diabetes and cardiovascular disease (12). So, we wondered how vaspin and lipocalin-2 levels, which are closely related with glucose metabolism alter in patients with OSAS who have an increased glucose intolerance and obesity incidence. Therefore, in this study we aimed to measure the levels of vaspin and lipocalin-2 which are secreted from adipocytes in patients with severe OSAS and examine the relationship between these two adipocytokines and OSAS.
Materials and methods
Study group
Patients were selected from polysomnography (PSG) studies at Bezmialem Foundation University Sleep Laboratory in a duration of nine months. Patients whose ages were between 25 and 65 with recently diagnosed severe OSAS with an apnea-hypopnea index (AHI) >30/h and healthy volunteers with an AHI <5/h were included into our study. Patients with diabetes mellitus, malignancy, chronic renal disease, chronic liver disease, psychiatric disorders, uncontrolled hypertension, coronary artery and cerebrovascular disease, patients with sleep disorders other than OSAS such as upper airway resistance syndrome, periodic leg movement syndrome, or narcolepsy and pregnants were excluded from the study. All volunteers underwent a thorough pysical examination and their height, weight, waist circumference and neck circumference were recorded. Weight and height were measured to the nearest kilogram and centimeter, respectively, and body mass index (BMI) was calculated [BMI = weight/(height)2]. Neck circumference was measured at the cricothyroid level, waist circumference in the middle between the 12th rib, and the iliac crest by a measure tape. Fasting glucose level, urea, creatinine, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting serum insulin level, HbA1c (glycated hemoglobin), complete blood count, free T3, free T4 and TSH were analyzed in all subjects. Serum of subjects was stored at –80 °C in order to measure vaspin; lipocalin-2 levels. A written consent from all subjects and the approval of the ethics committee of the Foundation University of Bezmialem were obtained.
Polysomnography (PSG)
A Compumedics E 3142 PSG device was used (Compumedics Inc., Melbourne, Australia). PSG findings were evaluated based on the guidelines published by American Academy of Sleep Medicine (AASM) in 2007, and the diagnosis of OSAS was confirmed (13). The average number of episodes of AHIwas calculated. Apnea was defined as complete cessation of airflow ≥10 s. Hypopnea was defined as a reduction of more than 50% of three respiratory signals, airflow signal or either respiratory or abdominal signals of respiratory inductance plethysmography, with an associated decrease of ≥3% in oxygen saturation or an arousal. OSAS was defined as an AHI ≥5/h with associated symptoms (sleep attacks or excessive daytime sleepiness), unsatisfying sleep, fatigue or insomnia, or heavy snoring and/or breathing pauses reported by the subject’s partner or an AHI ≥15/h regardless of associated symptoms. OSAS was defined as an AHI of ≥5/h plus clinical symptoms. Patients with an AHI of ≥30/h were included in OSAS group (patients with severe OSAS, n=34) and subjects with an AHI of <5/h were included in control group (normal controls, n=25). The PSG data was scored by three investigators.
Blood assay
Serum of all subjects was obtained from the venous blood samples taken in tubes with gel seperator between 08:00-09:00 hours in the morning after an average of 12 hours of fasting by centrifugation 3,600 rev/min for 10 minutes. The homeostasis model assessment insulin resistance index (HOMA-IR), as a measure of insulin sensitivity, was calculated as fasting insulin concentration (µu/mL) × fasting glucose concentration (mmol/L)/22.5 (14).
Serum of the subjects for the measurement of vaspin and lipocalin-2 was transferred into eppendorf tubes and stored at –80 °C until the day of the analysis. On the study day, vaspin ve lipocalin-2 levels were measured from the samples reached room temperature using a commercial enzyme immunoassay kit (Biovendor, Modrice, Czech Republic) according to the manufacturer’s instructions in an analyzer brand named Thermo Scientific Multiskan FC (USA). Samples were measured in duplicate, and the average was used in the data analysis.
Statistical analysis
Statistical Package for Social Sciences (SPSS) for Windows 20.0 software was used to perform the statistical analysis of the data. The continuous variables were expressed as mean ± standard deviation. One sample Kolmogrov-Sminov test was done to see if the continuous independent variables were normally distributed. Normally distributed independent continuous variales were compared student t-test whereas non-normally distributed independent continuous variables were compared with Mann-Whitney U test between two groups. Chi-square test was used for categorical variables. Bivariate correlation analyses were done by Spearman’s test. A P value<0.05 was considered statistically significant.
Results
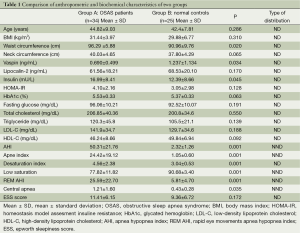
A total of 59 participants (34 from severe OSAS group of whom 8 were women; 25 from healthy volunteers of whom 10 were women) completed the study. There was no statistical difference between OSAS group (patients with severe OSAS group; AHI =49.4±23.1 events/h of sleep) and control group (Healthy volunteers; AHI =2.32±1.26 events/h of sleep) by means of sex, age, BMI, (P=0.175, P=0.286, P=0.31, respectively) (Table 1). There was no difference between OSAS group and control group in terms of lipocalin-2 levels while vaspin levels were found to be lower in OSAS group (P=0.34) (Table 1). Fasting serum insulin levels in OSAS group were significantly higher (P=0.045). There was no difference between the two groups in terms of fasting glucose, triglycerides, total cholesterol, LDL-C, HDL-C, HbA1c and HOMA-IR (Table 1).
Full table
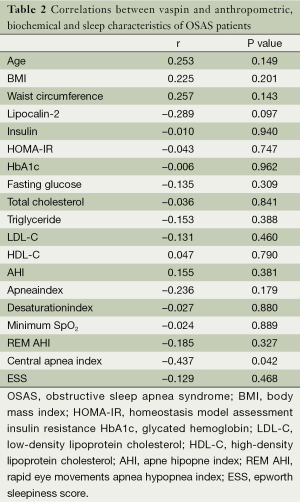
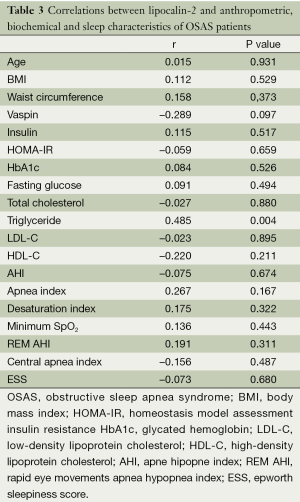
The correlation between vaspin and lipocalin-2 levels and anthropometric and biochemical characteristics of the patients with severe OSAS was also evaluated. A significant negative correlation between vaspin levels and central apnea index was found. A positive correlation was also found between serum levels of vaspin and lipocalin-2 but this correlation has not reached statistical significance. Finally, a significant positive correlation was found between triglyceride levels and lipocalin-2 levels in patients with severe OSAS (Tables 2,3). PSG results of each group were presented in Table 1.
Full table
Full table
Discussion
Vaspin and lipocalin-2 are recently identified members of adipocytokine family. In this study we measured serum levels of vaspin and lipocalin-2 in patients with OSAS which was found to be closely associated with obesity and insulin resistance. We also aimed to investigate the relationship between these adipocytokines and sleep and metabolic characteristics. Study groups included into our study did not differ in terms of sex, age, BMI, HOMA-IR, HbA1c, and neck circumference. Only waist cimcumference was significantly higher in OSAS group compared with healthy volunteers. We postulated that a linear association might exist between severity of OSAS and serum vaspin and lipocalin-2 levels so we included severe patients with AHI ≥30 events /h of sleep expecting more prominent results.
We failed to find a research paper analyzing vaspin and lipocalin-2 in patients with OSAS. Relatively few studies with other adipocytokines exist. In this study we report a significantly lower vaspin level in OSAS patients.
Previous studies showed a correlation between high serum vaspin level and obesity and high insulin resistance (15,16). However, this correlation between high serum vaspin level and obesity and high insulin resistance deteriorated in patients with uncontrolled type 2 diabetes and it was shown that serum vaspin levels decreased in these patients (15). Byung-Joon Ko et al. evaluated prepubertal 168 boys vs. 178 girls and found that serum vaspin levels increased significantly in subjects with obesity and high HOMA-IR (16). In our study groups were similar in terms of obesity and HOMA-IR leading a better assesment of effects of OSAS on vaspin levels.
Wang et al. compared 192 patients with OSAS and 144 healthy controls in terms of serum levels of omentin-1 which is an important member of the adipokine family and found significantly lower levels of omentin-1 in patients with OSAS (17). Recurrent episodes of hypoxia in OSAS lead to the release of various adhesion molecules and levels of inflammatory markers such as TNF, IL-6, CRP increases (18). Previously, it was shown that omentin-1 levels decreased in proinflammatory states (19). The finding that omentin-1 levels decreased in OSAS patients may be attributed to the secretion of these proinflammatory markers in OSAS. Similarly, recurrent episodes of hypoxia and increased oxidative stress may have led to a lower serum vaspin level in our study.
Trakada et al. compared severe OSAS patients with healthy controls in terms of serum visfatin levels, an another member of the adipocytokine family and found no difference among two groups. They also found a higher HOMA-IR and insulin levels in severe OSAS patients compared with healthy controls (20). Similarly, Makino et al. compared 230 patients with mild, moderate and severe OSAS patients in terms of levels of an another adipocytokine, adiponectin and insulin resistance. Adiponectin was shown to have an insulin resistance lowering effect and was found to be decreased in patients with obesity and high insulin resistance (21). While they found increased HOMA-IR levels in severe OSAS patients, they found no difference in terms of adiponectin levels among these three groups (22). In these studies, the compared groups differed in terms of insulin resistance which may have confounded the effects of OSAS on these markers. In our study, HOMA-IR levels were similar in both two groups, severe OSAS group and healthy controls group.
In our study a significant negative correlation between vaspin levels and central apnea index was found. If this finding is supported by larger studies, vaspin may reveal itself as a valuable marker in suspecting a high central apnea index.
There was no significant difference among patients with OSAS and healthy controls in terms of serum levels of lipocalin-2 in our study. No study investigated lipocalin-2 levels in OSAS before but there are studies showing higher lipocalin-2 levels with higher BMI and insulin resistance and lower levels of lipocalin-2 in patients under treatment reducing insulin resistance such as thiazolidinediones (23,24). This difference may be attributed to different underlying pathophysiological mechanisms leading to obesity, insulin resistance and OSAS. Our finding of an inverse relationship between lipocalin-2 and triglycerides is coherent with the findings of previous studies.
The limitation of our study included small sample size, exclusion of patients with mild and moderate OSAS, lack of measurement of serum vaspin and lipocalin-2 levels after continuous positive airway pressure (CPAP) treatment.
Conclusions
In conclusion, we found that serum vaspin levels were lower in severe OSAS patients compared to healthy controls and lipocalin-2 levels did not differ among these groups. The decrease in serum vaspin levels in severe OSAS patients may be important in diagnosis and follow-up of these patients. A study investigating the change in vaspin levels after CPAP treatment may yield important results. Further studies with a larger sample are needed to confirm our results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bixler EO, Vgontzas AN, Ten Have T, et al. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med 1998;157:144-8. [PubMed]
- Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163:608-13. [PubMed]
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217-39. [PubMed]
- Deegan PC, McNicholas WT. Pathophysiology of obstructive sleep apnoea. Eur Respir J 1995;8:1161-78. [PubMed]
- Grunstein R, Wilcox I, Yang TS, et al. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord 1993;17:533-40. [PubMed]
- Lattimore JD, Celermajer DS, Wilcox I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol 2003;41:1429-37. [PubMed]
- Coughlin SR, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J 2004;25:735-41. [PubMed]
- Matsuzawa Y, Funahashi T, Kihara S, et al. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 2004;24:29-33. [PubMed]
- Hida K, Wada J, Eguchi J, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A 2005;102:10610-5. [PubMed]
- Wang Y, Lam KS, Kraegen EW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem 2007;53:34-41. [PubMed]
- Choi KM, Lee JS, Kim EJ, et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol 2008;158:203-7. [PubMed]
- Kim NH, Cho NH, Yun CH, et al. Association of obstructive sleep apnea and glucose metabolism in subjects with or without obesity. Diabetes Care 2013;36:3909-15. [PubMed]
- Iber C, Ancoli IS, Chesson AL, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. American Academy of Sleep Medicine, Westchester, 2007.
- Bonora E, Targher G, Alberiche M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;23:57-63. [PubMed]
- Youn BS, Klöting N, Kratzsch J, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008;57:372-7. [PubMed]
- Ko BJ, Lee M, Park HS, et al. Elevated vaspin and leptin levels are associated with obesity in prepubertal Korean children. Endocr J 2013;60:609-16. [PubMed]
- Wang Q, Feng X, Zhou C, et al. Decreased levels of serum omentin-1 in patients with obstructive sleep apnoea syndrome. Ann Clin Biochem 2013;50:230-5. [PubMed]
- Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003;107:1129-34. [PubMed]
- Shibata R, Ouchi N, Kikuchi R, et al. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis 2011;219:811-4. [PubMed]
- Trakada G, Steiropoulos P, Nena E, et al. Plasma visfatin levels in severe obstructive sleep apnea-hypopnea syndrome. Sleep Breath 2009;13:349-55. [PubMed]
- Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr 2009;4:44-9. [PubMed]
- Makino S, Handa H, Suzukawa K, et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol (Oxf) 2006;64:12-9. [PubMed]
- Wang Y, Lam KS, Kraegen EW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem 2007;53:34-41. [PubMed]
- Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007;56:2533-40. [PubMed]


