
Endobronchial ultrasound-guided transbronchial needle aspiration mediastinal lymph node staging in malignant pleural mesothelioma
Introduction
Malignant Pleural Mesothelioma (MPM) is an aggressive tumor with poor survival. Management depends on tumor type, extent of the disease and patient comorbidities. Minority of patients can be managed with a multimodality therapy with improved survival (1-3). Patients with intrathoracic or distant nodal metastases managed surgically have worse clinical outcomes than patients with no nodal metastases (1,2). Therefore, multimodality management requires a careful patient selection to ensure no distant or intrathoracic nodal metastases. Sensitivity of computed tomography (CT) and positron emission tomography CT (PET-CT) in detecting nodal metastases is limited (4-11). Surgical invasive staging [cervical mediastinoscopy (CM), or transcervical extended mediastinal lymphadenectomy (TEMLA)] have poor sensitivity and procedure-related morbidity, for these reasons, these techniques are not commonly implemented as part of patient assessment (8,12). Improved detection of nodal metastases is needed pre-operatively, given high prevalence of nodal metastases in patients managed surgically (13). Use of endoscopic techniques [endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and esophageal ultrasound-guided fine needle aspiration (EUS-FNA)] for mediastinal staging, has been reported by some mesothelioma programs but limited literature exists on their performance in patients with MPM (8,12,14). The current study reports on performance of EBUS-TBNA in intrathoracic lymph node (LN) staging in patients with MPM and low prevalence of intrathoracic nodal metastases, managed at a large MPM program at a tertiary thoracic surgery cancer center.
Methods
We performed a retrospective chart review of patients referred to the MPM program at the University Health Network (UHN), a tertiary thoracic oncology center in Canada, for consideration of trimodality therapy between January 1, 2012 and December 31, 2014 who underwent EBUS-TBNA mediastinal LN staging as part of pre-operative evaluation. Trimodality approach includes accelerated hemithoracic intensity-modulated radiation, followed by extrapleural pneumonectomy (EPP) according to the Surgery for Mesothelioma After Radiation Therapy (SMART) protocol developed by the UHN malignant mesothelioma program (1). MPM program at the UHN is a national referral center for management of patients with MPM.
Patients eligible for the SMART approach were at least 18 years of age and had an Eastern Cooperative Oncology Group performance status of 0 to 2, with good pulmonary function tests (defined as forced expiratory volume in 1 second >40% predicted or diffusing capacity for carbon monoxide >45% predicted), a new histological diagnosis of MPM previously untreated, clinical stage T1-3N0M0, suitable for combined modality therapy, and able to give informed consent. Clinical stage was determined by high-resolution CT scan of the chest and abdomen, fluorodeoxyglucose (FDG)-PET/CT scan, and brain magnetic resonance imaging (MRI) or CT. Histological diagnosis and staging was based on the 2004 World Health Organization classification system and the seventh edition of the TNM staging system (15,16).
EBUS-TBNA
EBUS-TBNA was performed using the convex probe endobronchial ultrasound (CP-EBUS) (BF-UC180F-OL8, Olympus, Tokyo, Japan). The ultrasound image was processed in a dedicated universal ultrasound processor (EU-ME1, Olympus, Tokyo, Japan). Static ultrasound images were obtained, and size of LNs measured in 2 dimensions. Doppler mode imaging was used selectively. All procedures were conducted in an endoscopy suite and using intravenous conscious sedation with midazolam and fentanyl. Conventional flexible bronchoscopy was performed first, followed by examination of the mediastinum using the CP-EBUS. The location and size of the LNs (ipsilateral and contralateral) were characterized and classified as N1, N2, or N3. A dedicated 22-gauge needle (NA-201SX-4022, Olympus) was used to perform all EBUS-TBNA procedures as previously described (17,18). For the assessments that were performed with the presence of Rapid on Site Evaluation (ROSE) the internal stylet was used to push the specimen out onto a slide for cytologic examination, followed by a needle rinse in sterile saline. Smears were air dried and fixed in modified Carnoy’s solution. The air-dried smears were stained with a modified Field’s stain and, where available, evaluated by an on-site cytopathologist to confirm “adequate” cell material. Adequate cell material was defined as sufficient material for a specific diagnosis or the presence of lymphocytes in the specimen (19). LN sampling was deemed as “negative” if sample provided demonstrated adequate lymphoid material with no evidence of malignant cells.
LN sampling that yielded inadequate lymphoid material, or no lymphoid material and no evidence of metastatic tumor in the sample provided was deemed “non-diagnostic”.
Contralateral LNs were sampled first followed by midline or ipsilateral N2 LNs. Where multiple nodes were seen, the most suspicious node in each group was targeted first. We used Fujiwara’s criteria to define suspicious LNs. Suspicious LNs were defined as round, well demarcated, echo-poor, with short axis of more than 1 cm and FDG avid (SUV >2.5) (20,21). The number of passes per LN and per patient, was at the discretion of the bronchoscopist. If ROSE was available, the number of passes was guided by the feedback of the cytopathologist. If LN was reported as positive, further passes were not pursued. If sample was suspicious for metastatic involvement, further passes were performed at the LN until adequate sample was obtained or until cytopathologist indicted that subsequent samples provided were of deteriorating quality and therefore not contributory. If samples provided were deemed satisfactory but negative for tumor by the cytopathologist, a minimum of two passes were performed to ensure that different locations of the LN were sampled. When ROSE was not available, three passes were performed at each sampled LN (22,23).
If a sample examined by the on site cytopathologist was deemed positive or suspicious for tumor cells, a new needle was used to sample other LNs (if further sampling was conducted). For the cases where ROSE was not available, different needles were used to sample enlarged (short axis diameter >1 cm), FDG avid (SUV >2.5) LNs, or LNs with sonographic features suspicious for metastasis to prevent cross-contamination. If a nodal station did not contain enlarged, FDG-avid or LNs with sonographic features suggestive of malignancy, the most accessible LN was sampled.
LN localization was described according to the 7th TNM classification for lung cancer (24). EBUS-TBNA was performed for all EBUS-TBNA accessible, enlarged (short axis >1 cm), FDG avid (SUV >2.5) LNs and LNs with sonographic features suggestive of malignancy (as described above).
A modified Papanicolaou stain was used for the Carnoy’s fixed slides. The needle rinse was processed by cell block or ThinPrep® CytoLyt® slide production, and light microscopy was carried out by a cytopathologist. For the samplings where ROSE was not available, all needle contents were rinsed into a ThinPrep®, CytoLyt® (alcohol fixative) and processed into a cell block in the lab for examination under light microscopy by the cytopathologist.
EBUS-TBNA was performed by a thoracic surgeon (K Yasufuku) or an interventional pulmonologist (K Czarnecka-Kujawa).
Contralateral LNs were sampled first followed by midline or ipsilateral N2 LNs. Where multiple nodes were seen, the most suspicious node in each group was targeted first. Suspicious nodes were defined as round, well demarcated, and echo-poor (20). If a sample examined by the on site cytopathologist was deemed positive or suspicious for tumor cells, a new needle was used to sample other LNs (if further sampling was conducted). For the cases where ROSE was not available, different needles were used to sample enlarged (short axis diameter >1 cm), FDG avid (SUV >2.5) LNs, or LNs with sonographic features suspicious for metastasis to prevent cross-contamination.
LN localization was described according to the 7th TNM classification for lung cancer (24). EBUS-TBNA was performed for all EBUS-TBNA accessible, enlarged (short axis >1 cm), FDG avid (SUV >2.5) LNs and LNs with sonographic features suggestive of malignancy (as described above).
Therapy
Patients with no distant metastasis and negative EBUS-TBNA staging underwent hemithoracic radiation, followed by EPP according to the SMART protocol (1). SMART entails a total of 25 Gy of radiation delivered in 5 daily fractions over 1 week to the entire ipsilateral hemithorax by intensity-modulated radiation therapy (IMRT), with a concomitant boost of 5 Gy to volumes at high risk based on CT and PET scan findings. EPP was performed within 2 weeks after the end of radiation therapy before the development of radiation pneumonitis. Adjuvant chemotherapy with cisplatin and an antifolate (pemetrexed or raltitrexed) doublet were administered selectively to patients with ypN2 disease on final pathology (1). All patients who underwent EPP underwent mediastinal LN dissection.
The study was approved by institutional ethics board of UHN (No. 14-7882).
Statistical analysis
If the LN sample was positive for metastatic mesothelioma or other cancer, the results were considered “true positive”. In cases of negative EBUS-TBNA staging, pathological staging obtained during EPP, was considered “gold standard”. LNs negative on EBUS-TBNA and on final surgical pathology were deemed “true negatives”. Only cases where patients underwent the EPP and surgical lymphadenectomy were included in calculation of EBUS-TBNA performance characteristics [sensitivity, negative predictive value (NPV), positive predictive value (PPV) and diagnostic accuracy]. Statistical analysis was performed using the student t-test for comparison of continuous variables, the Chi-square or Fisher exact test for comparison of dichotomous outcomes, as appropriate. All hypothesis testing was two-sided, using an alpha level of 0.05. Analysis was conducted using Microsoft Excel (Microsoft Office 365 ProPlus Version 15.0.4911.1002).
Results
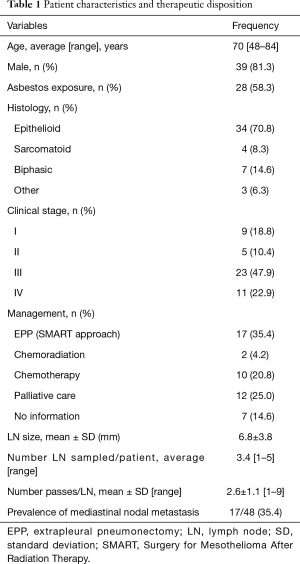
Forty-eight patients were referred to our program in the study timeframe and all were eligible for analysis (i.e., all patients referred were staged invasively as part of assessment and they were staged with EBUS-TBNA only). Average age was 70 years (range, 48–84 years). Male prevalence was 81.3% (n=39). Asbestos exposure was present in 28 (58.3%) of patients. Tumor histology was as follows: epithelioid 34/48 (70.8%), biphasic 7/48 (14.6%), sarcomatoid 4/48 (8.3%), other 3 (6.3%) (1 patient with pleural biopsy positive for desmoplastic mesothelioma; 1 patient with pleural pathology consistent with either epithelioid or biphasic tumor, conclusive diagnosis was not possible; 1 patient with pleural biopsies suspicious for mesothelioma and with p16 mutation but further characterization of the tumor not possible). Clinical stage was assigned according to the TNM malignant mesothelioma classification, 7th edition (15). The stage distribution was as follows: stage I 9/48 (18.8%), stage II 5/48 (10.4%), stage III 23/48 (47.9%), stage IV 11/48 (22.9%). Seventeen patients were managed with EPP (35.4%). Twelve patients managed with EPP had epithelioid mesothelioma, while 5 surgical patients had biphasic mesothelioma. Combined chemotherapy and radiation were used in 2 patients (4.2%). Ten patients (20.8%) had chemotherapy alone. Symptom palliation was undertaken in 12 patients (25.0%). Care of 7 patents (14.6%) was transferred back to their home provinces after patients were deemed not to be surgical candidates (Table 1).
Full table
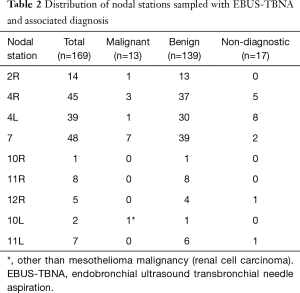
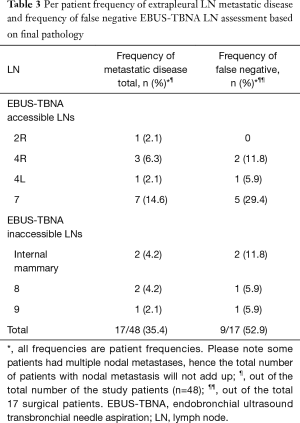
Total of 169 LNs were sampled. Diagnostic tissue was obtained from all patients in whom EBUS-TBNA was performed. Mean sampled LN size was 6.8±3.8 mm. On average 3 LNs/patient (range, 1–5) we sampled. Diagnostic sample was obtained from 152 LNs (89.9%). Mean diagnostic LN size was 7.8±4.1 mm. Seventeen sampled LNs were non-diagnostic (10.1%) (Table 2). The mean non-diagnostic LN size was 5.6±1.1 mm. The non-diagnostic LNs were significantly smaller than the diagnostic LNs (P=0.0362). Of the 48 patients staged invasively with EBUS-TBNA, nodal metastasis was detected in 9 patients (18.8%). Metastatic mesothelioma was detected in 8 patients. Metastatic renal cell carcinoma was diagnosed in one patient. Mean sampled malignant LN size was 13.9±6.2 mm. On average, malignant LNs were larger than benign LNs (P<0.0001). Trimodally therapy was performed on 17 patients with no nodal metastasis on EBUS-TBNA (35.4%). Nodal metastases were detected on final pathology in 9/17 patients (52.9%). Prevalence of nodal metastasis due to mesothelioma in the cohort was 17/48 (35.4%) (Table 3). Sensitivity, specificity, NPV, PPV and diagnostic accuracy of preoperative EBUS-TBNA mediastinal LN staging in MPM was 16.7%, 100%, 100%, 68.8% and 70.6%, respectively. Metastasis in LNs inaccessible to EBUS occurred in 4/17 (23.5%) of the surgical patients (two patients with metastasis in internal mammary LNs, and in station 8 and 9, in two separate patients) and accounted for 44% of false negative staging. Frequencies of nodal material obtained from different stations are presented in Table 2. The average waiting time from the staging EBUS-TBNA to EPP was 49±33 days. The waiting time between the EBUS-TBNA staging and the surgery for patients whose EBUS-TBNA results were concordant (in EBUS-TBNA accessible LNs) with the final pathology and those whose EBUS-TBNA staging was discordant with the final pathology was 38±26 and 76±38 days, respectively, P=0.00292. The difference remained significant when patients with biphasic mesothelioma were excluded from the calculation.
Full table
Full table
Discussion
Nodal metastasis is a negative prognostic factor in MPM, associated with reduced survival. Aggressive multimodality therapy involving a combination of surgery (i.e., EPP or pleurectomy/decortication) with hemithoracic radiation and chemotherapy has been shown to improve survival in a subset of patients with epithelioid mesothelioma, low disease bulk and no nodal metastasis (pN0 status) (mean survival of 24–26 vs.16–17 months in patients with nodal disease) (10,25). Recently, de Perrot and colleagues have shown favourable disease-free survival of 66% at 3 years, in patients with pN0 status managed with neoadjuvant hemithoracic radiation, followed by EPP, compared to 48% 3-year survival in patients with pN+ disease (1). Friedberg et al. showed an impressive 7.3-year overall and 2.3-year disease-free survival in patients with no nodal metastasis who were treated with extended pleurectomy-decortication and intraoperative photodynamic therapy (3). Patients with nodal metastasis detected on final pathology have survival half of that seen in patents with no nodal metastasis (1,2,7,10,26-29). Unfortunately, nodal metastasis is present in 13–50% of patients managed surgically (1,8,10).
Accurate preoperative staging is key to direct management of patients with MPM, to improve survival while avoiding mortality and morbidity associated with multimodality treatments.
At present, preoperative staging with CT and PET/PET-CT are commonly utilized as non-invasive staging strategies. CT chest can detect contralateral, distal metastasis, or gross chest wall invasion. However, CT has been shown to understage patients with focal tumor invasion of the chest wall, pericardium or diaphragm. There is no correlation between presence of intrathoracic lymphadenopathy detected on CT chest and nodal metastasis. Nodal enlargement is neither sensitive nor specific as an indicator of nodal metastasis and interlobar and hilar LNs may be difficult to distinguish from the adjacent tumor (6,9). In addition, there is a significant inter-observer variability in interpretation of CT chest finding and reported CT-based staging (30). Overall, CT sensitivity for detection of intrathoracic nodal metastasis is reported at 60% (6).
PET and PET-CT have shown improvement in detection of local and distant metastasis (5,11) with sensitivity for detection of T4 disease and N2/N3 disease of 78% and 50%, respectively. Reported PET-CT sensitivity for detection of metastasis ranges between 11–83%. (4,5). Erasmus et al. reported a false negative PET in some patients with N1 metastasis due to presence of confluent tumor within the interlobar region. In addition, some patients were upstaged as having N3 disease based on PET-CT, with later pathologic confirmation of false positives due to inflammation (5). In addition, metastases have been reported in LNs as small as 4 mm, which is below PET-CT resolution (8).
Novel CT chest and PET-CT imaging parameters have been proposed as tools to predict the presence or absence of metastatic disease or even post-treatment prognosis. For example, tumor thickness has been proposed as a surrogate of tumor volume or tumor burden which, in combination with rind-like morphology, were shown to be associated with LN metastasis and with overall patient survival following therapy (3,10,31,32). In addition, studies assessing the utility of integrated PET-CT have shown that the degree of tumor FDG avidity, total tumor glycolysis and mean tumor volume correlate positively with presence of metastatic disease and with reduced survival post-treatment (33,34). Although these radiographic features may be useful prognostic factors, their use in disease staging, estimating probability of nodal metastasis and survival, have not been validated, and as such, imaging alone cannot be relied on for therapeutic decision-making in MPM. Positive CT chest or PET-CT findings should be correlated pathologically.
Invasive intrathoracic nodal staging in MPM has been proposed by several authors (8,12). However, despite high prevalence of nodal metastasis in patients undergoing surgical management of MPM, and well documented poor outcomes in patients with nodal metastasis managed surgically, only minority (~38%) of patients undergoing surgical management receive invasive intrathoracic staging (10).
There are several issues related to invasive nodal staging in patients with MPM that may be responsible for low implementation rate. The two main issues relate to interpretation of prognostic information collected for the past 20 years, and the N classification of TNM staging in MPM based on the lung cancer map (35), which assumes that tumors invade pulmonary lymphatics, drain progressively through intraparenchymal and ipsilateral hilar LNs (N1 LNs) to ipsilateral and midline mediastinal nodes (N2 LNs), and finally to contralateral and extrathoracic stations (N3 LNs). Nodal invasion in MPM has some nuances, which are not accounted for in the lung cancer nodal map. For example, although MPM invading into pulmonary parenchyma may follow lung cancer metastatic pattern, direct lymphatic drainage from the diaphragmatic pleura to the mediastinal nodal chain can occur (36) most likely accounting for skip metastasis (N2 nodal metastasis without N1 involvement) documented in as many as 51% of patients (10,37-39). Prognostic value of nodal involvements in MPM, has not been evaluated to the same extent as in lung cancer. Second issue is that many intrathoracic LNs that can be involved in mesothelioma (i.e., internal mammary, pericardial fat) are not accessible to conventional staging with CM and the modern endoscopic techniques, limiting utility of invasive preoperative staging.
CM and TEMLA as part of a staging algorithm have been proposed for invasive mediastinal staging in MPM (6,8,9,12,26,37,38,40). CM performance in MPM has been associated with low sensitivity and high false negative rate ranging between 28–36% and 22–53%, respectively. One of the reasons for poor CM performance in MPM is that CM, cannot access many LNs shown to be involved commonly in MPM, for example internal mammary [highest frequency of metastasis (41%) on per LN basis], peridiaphragmatic, pericardial, retrocrural, intercostal, hilar (10). Station 7 LN, has been shown to be most commonly reported as positive on invasive staging in MPM (36% of patients), but with only 50% of these metastases detected on invasive pre-operative staging (8). This may be because CM can only access the anteriorly and caudally located station 7 LN with no access to posteriorly located station 7 LN (41,42).
CM can be associated with low, although not insignificant, mortality and morbidity, which include a potentially catastrophic injury to major vessels, recurrent laryngeal nerve, esophagus and tracheobronchial tree (43,44). The use of TEMLA has been studied for mediastinal LN staging in patients with MPM. Even though TEMLA can pick up additional metastasis not detected by endoscopic testing, the procedure has a high rate of complications (6.0–13.2%) and mortality rate of up to 1.2%. Some studies reported that as many as 20% of qualifying lung cancer patients staged with TEMLA were unable to undergo surgical management of their cancer due to deterioration of their clinical status post procedure (45-49).
Development of endoscopic techniques of EBUS-TBNA and EUS-FNA with better safety profile (50) and similar or better diagnostic yield than the surgical techniques in lung cancer staging (19,42), prompted some mesothelioma programs to use these techniques to stage the mediastinum in patients with MPM (8,12). Rice et al. showed better sensitivity and NPV of EBUS-TBNA compared to CM for intrathoracic staging in MPM, 58% vs. 28% and 49% vs. 57%, respectively, in a patient population with a high prevalence of intrathoracic nodal metastasis (8). The sensitivity of EBUS-TBNA in our study was lower (16%) than that demonstrated by Rice, although diagnostic samples were obtained in nearly 90% of sampled LNs and none of the non-diagnostic LNs (17/169) were found to be positive on the final pathology. Low test sensitivity could be related to a low prevalence of intrathoracic nodal metastasis related to mesothelioma in our cohort (35.4%), presence of nodal metastasis in EBUS-TBNA inaccessible LNs [4/17 (23.5%) of patients with nodal metastasis] and its impact given the study’s small sample size. EBUS-TBNA staging prevented unnecessary surgical intervention in 18.8% of patients (9/48) without associated additional patient morbidity and only at a fraction of a cost associated with surgical treatment (51). Zielinski suggested a combination of endoscopic (EBUS-TBNA and EUS-FNA staging) and surgical stating (TEMLA) in patients with MPM, showing that test combination detected nodal metastasis in 77.7% of their patients (12). Similarly, improved diagnostic yield in mediastinal LN sampling has been shown with a combination of surgical and non-surgical techniques in lung cancer staging (52). However, in the Zielinski’s cohort, all but one positive LNs identified on TEMLA, were accessible to EBUS-TBNA, arguing that additional needle passes with EBUS-TBNA might have been sufficient to identify metastasis. Other authors also demonstrated that majority of positive LNs are in EBUS-TBNA accessible stations (60%) (8). Previous studies of endoscopic staging in lung cancer have shown that multiple passes are required for adequate diagnostic tissue acquisition, including for molecular analysis (22,53). This suggests that improved diagnostic yield seen with a combination of techniques may be related to larger amount of tissue acquired per LN, rather than to limitation of exclusive EBUS-TBNA invasive staging. Given a less invasive nature of endoscopic staging, with similar LN access to TEMLA, use of TEMLA may not be justifiable in patients with negative endoscopic staging, instead a more detailed LN assessment should be performed by EBUS-TBNA.
Despite invasive staging, 9/17 (52.9%) patients in our cohort who underwent EPP had nodal disease identified at final pathology. This finding is consistent with previously reported literature (10). One of the reasons for this could have been the time interval between the staging EBUS-TBNA and EPP (mean waiting time for surgery of 49±33 days). Given the significantly longer waiting time for surgery in patients with discordant with the pre-operative EBUS-TBNA staging results on the final pathology, it is possible that nodal metastasis occurred following the staging EBUS-TBNA. This observation suggests that timing of the surgery following invasive staging may be an important factor in optimizing patient care in MPM. More studies are necessary to further investigate the safe time interval from staging to curative surgery, after which, staging should be repeated to ensure no disease progression. In addition, involvement of EBUS-TBNA inaccessible LNs was present in 23.5% of patients with nodal metastasis. Eleven point eight percent (2/17 patients) of patients had metastasis in EUS-FNA accessible LNs only (station 8 and 9) with no metastasis in EBUS-TBNA accessible LNs. Performing a selective EUS-FNA, as suggested by other authors, may improve performance of endoscopic staging in MPM (8,54).
Reported disease-free survival and overall survival in patients with pN+ disease managed surgically is 18 and 51 months, respectively. In contrast, median survival of 8 months (4 months with palliative management only, and 14 months with chemotherapy) has been reported in MPM patients managed non-surgically (55). A direct comparison of survival in patients with MPM and micrometastatic nodal disease managed with multimodality approach vs. chemotherapy alone is not available and multiple factors play a role in patient survival (including T, N and M tumor stage, tumor type, patient age). Patients managed with non-surgical approaches have clinically and pathologically more advanced disease than patients managed surgically. Nonetheless, the population of patients with micrometastatic nodal metastasis may be different in terms of tumor biology as compared to patients with macrometastatic disease. Further studies of long-term survivors among surgically and non-surgically managed patients with MPM and nodal disease may shed light on tumor-specific factors responsible for less aggressive disease and better outcomes in this patient population.
Our study had several limitations. It is a retrospective study of a select group of patients with low prevalence of nodal metastasis with all procedures performed by expert endoscopists, therefore, these results may not be applicable to other programs. In addition, the time interval between the staging procedures and the surgery, might have allowed for disease progression leading to discordance between the EBUS-TBNA and the surgical pathology results, lowering EBUS-TBNA performance characteristics.
Lastly, given retrospective nature of the study, there was no standardization of CT chest and PET-CT procedure preparation, execution and reporting protocols. For the CT chest, all scans were reviewed individually and intrathoracic LNs assessed and measured if lymphadenopathy was suspected by the study principal investigator (PI). For PET-CT standard SUV >2.5 was used to define significant FDG-avidity, limiting reporting variability.
Modifications to the MPM TNM stage grouping were introduced in 2016. The latest, 8th edition of the TNM staging in Malignant Mesothelioma proposed by the International Association for the Study of Lung Cancer (IASLC), has been modified to include nodal-depended outcomes based on data from a large, international database of patients with MPM who had their mediastinum staged invasively (10). Prognostic data from this database led to unification of the previous N1 (ipsilateral segmental, interlobar and hilar LNs), with the previous N2 LNs (subcarinal, ipsilateral paratracheal, peridiaphragmatic, internal mammary, intercostal and pericardial fat pad) into one common N1 category. Contralateral N1 and N2 LNs including ipsilateral and contralateral supraclavicular LN are now classified as N2 category (10). The new stage groupings based on the N component, may now be used more reliably to make treatment recommendations and to offer prognostic information. Involvement of one intrathoracic LN is enough to preclude surgery. However, information on status of other intrathoracic LNs obtained as part of endoscopic staging, may be useful prognostic perspective. EBUS-TBNA with its access to N1 LNs can offer advanced information on the extent of nodal involvement. Even though there is no survival difference between patients with only N1 or only N2 LN metastasis, patients with both N1 and N2 metastasis fair worse than those with only N1 or only N2 LN involvement (10). This information could be used in several different ways: (I) to discuss prognosis with patients with different extent of nodal disease; (II) potentially to tailor non-surgical therapy in setting of clinical trials; (II) and hopefully to better understand survival differences among some patients with MPM. Even though we and previous investigators showed low sensitivity of the endoscopic mediastinal LN staging in patients with MPM, the staging prevented futile surgery in nearly 19% of patients in our study (a population of patients with low prevalence of nodal disease), and in as many as 50% in a population of patients with higher prevalence of nodal metastasis in previous study by Rice. Given the safety of the endoscopic staging, and morbidity and mortality of surgical management of MPM, we believe that despite its low overall sensitivity, endoscopic staging is useful and should be part of invasive staging in mesothelioma. Further studies are needed to explore the use of other clinical tumor parameters, based on CT and PET-CT findings, in predicting nodal metastasis; to determine optimal staging approach (i.e., one endoscopic modality vs. two) and safe surgery waiting time, to ensure low probability of disease progression and eliminate the need for repeated staging.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. K Czarnecka-Kujawa is a consultant to Olympus America; Dr. M de Perrot received personal fees from Bayer, Merck and Actelion, outside the submitted work; Dr. K Yasufuku is a consultant to Olympus America. S Keshavjee has no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Ethics Board of University Health Network (No. 14-7882).
References
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- Sugarbaker DJ, Strauss GM, Lynch TJ, et al. Node status has prognostic significance in the multimodality therapy of diffuse, malignant mesothelioma. J Clin Oncol 1993;11:1172-8. [Crossref] [PubMed]
- Friedberg JS, Simone CB 2nd, Culligan MJ, et al. Extended Pleurectomy-Decortication-Based Treatment for Advanced Stage Epithelial Mesothelioma Yielding a Median Survival of Nearly Three Years. Ann Thorac Surg 2017;103:912-9. [Crossref] [PubMed]
- Flores RM, Akhurst T, Gonen M, et al. Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2003;126:11-6. [Crossref] [PubMed]
- Erasmus JJ, Truong MT, Smythe WR, et al. Integrated computed tomography-positron emission tomography in patients with potentially resectable malignant pleural mesothelioma: Staging implications. J Thorac Cardiovasc Surg 2005;129:1364-70. [Crossref] [PubMed]
- Schouwink JH, Kool LS, Rutgers EJ, et al. The value of chest computer tomography and cervical mediastinoscopy in the preoperative assessment of patients with malignant pleural mesothelioma. Ann Thorac Surg 2003;75:1715-8; discussion 1718-9.
- Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999;68:1799-804. [Crossref] [PubMed]
- Rice DC, Steliga MA, Stewart J, et al. Endoscopic ultrasound-guided fine needle aspiration for staging of malignant pleural mesothelioma. Ann Thorac Surg 2009;88:862-8; discussion 868-9. [Crossref] [PubMed]
- Pilling JE, Stewart DJ, Martin-Ucar AE, et al. The case for routine cervical mediastinoscopy prior to radical surgery for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2004;25:497-501. [Crossref] [PubMed]
- Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2100-11.
- Elliott HS, Metser U, de Perrot M, et al. (18)F-FDG PET/CT in the management of patients with malignant pleural mesothelioma being considered for multimodality therapy: experience of a tertiary referral center. Br J Radiol 2018;91:20170814. [Crossref] [PubMed]
- Zielinski M, Hauer J, Hauer L, et al. Staging algorithm for diffuse malignant pleural mesothelioma. Interact Cardiovasc Thorac Surg 2010;10:185-9. [Crossref] [PubMed]
- Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. [Crossref] [PubMed]
- Tournoy KG, De Ryck F, Vanwalleghem LR, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med 2008;177:531-5. [Crossref] [PubMed]
- Sobin L, Gospodarowicz MK. TNM Classification of Malignant Tumors 7th edition. Oxford, UK: Backwell Publishing Ltd., 2009.
- Travis WS. Pathology and |Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon, Frnce: IARC Press, 2004.
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Koh E, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer 2005;50:347-54. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Fujiwara T, Yasufuku K, Nakajima T, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer: a standard endobronchial ultrasound image classification system. Chest 2010;138:641-7. [Crossref] [PubMed]
- Broderick SR, Patterson GA. Performance of integrated positron emission tomography/computed tomography for mediastinal nodal staging in non-small cell lung carcinoma. Thorac Surg Clin 2013;23:193-8. [Crossref] [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 2014;260:577-80; discussion 580-2. [Crossref] [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [Crossref] [PubMed]
- Bolukbas S, Manegold C, Eberlein M, et al. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/Pemetrexed and radiotherapy. Lung Cancer 2011;71:75-81. [Crossref] [PubMed]
- Minatel E, Trovo M, Polesel J, et al. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer 2014;83:78-82. [Crossref] [PubMed]
- Gomez DR, Hong DS, Allen PK, et al. Patterns of failure, toxicity, and survival after extrapleural pneumonectomy and hemithoracic intensity-modulated radiation therapy for malignant pleural mesothelioma. J Thorac Oncol 2013;8:238-45. [Crossref] [PubMed]
- Rusch VW, Gill R, Mitchell A, et al. A Multicenter Study of Volumetric Computed Tomography for Staging Malignant Pleural Mesothelioma. Ann Thorac Surg 2016;102:1059-66. [Crossref] [PubMed]
- Gill RR, Richards WG, Yeap BY, et al. Epithelial malignant pleural mesothelioma after extrapleural pneumonectomy: stratification of survival with CT-derived tumor volume. AJR Am J Roentgenol 2012;198:359-63. [Crossref] [PubMed]
- Liu F, Zhao B, Krug LM, et al. Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol 2010;5:879-84. [Crossref] [PubMed]
- Klabatsa A, Chicklore S, Barrington SF, et al. The association of 18F-FDG PET/CT parameters with survival in malignant pleural mesothelioma. Eur J Nucl Med Mol Imaging 2014;41:276-82. [Crossref] [PubMed]
- Kitajima K, Doi H, Kuribayashi K, et al. Prognostic value of pretreatment volume-based quantitative (18)F-FDG PET/CT parameters in patients with malignant pleural mesothelioma. Eur J Radiol 2017;86:176-83. [Crossref] [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Okiemy G, Foucault C, Avisse C, et al. Lymphatic drainage of the diaphragmatic pleura to the peritracheobronchial lymph nodes. Surg Radiol Anat 2003;25:32-5. [Crossref] [PubMed]
- Edwards JG, Stewart DJ, Martin-Ucar A, et al. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 2006;131:981-7. [Crossref] [PubMed]
- de Perrot M, Uy K, Anraku M, et al. Impact of lymph node metastasis on outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2007;133:111-6. [Crossref] [PubMed]
- Flores RM, Routledge T, Seshan VE, et al. The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: implications for revision of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2008;136:605-10. [Crossref] [PubMed]
- Rice D. Surgery for malignant pleural mesothelioma. Ann Diagn Pathol 2009;13:65-72. [Crossref] [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Lemaire A, Nikolic I, Petersen T, et al. Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate. Ann Thorac Surg 2006;82:1185-9; discussion 1189-90. [Crossref] [PubMed]
- Lardinos D, Weder W. Mediastinoscopy. Pearson FG, Cooper JD, Deslauriers J, et al. editors. Thoracic Surgery. 2nd edition. New York: Churchill Livingstone, 2002:98-103.
- Kuzdzal J, Zielinski M, Papla B, et al. The transcervical extended mediastinal lymphadenectomy versus cervical mediastinoscopy in non-small cell lung cancer staging. Eur J Cardiothorac Surg 2007;31:88-94. [Crossref] [PubMed]
- Kuzdzal J, Zielinski M, Papla B, et al. Transcervical extended mediastinal lymphadenectomy--the new operative technique and early results in lung cancer staging. Eur J Cardiothorac Surg 2005;27:384-90; discussion 390. [Crossref] [PubMed]
- Kuzdzal J, Szlubowski A, Grochowski Z, et al. Current evidence on transcervical mediastinal lymph nodes dissection. Eur J Cardiothorac Surg 2011;40:1470-3. [PubMed]
- Call S, Obiols C, Rami-Porta R, et al. Video-Assisted Mediastinoscopic Lymphadenectomy for Staging Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:1326-33. [Crossref] [PubMed]
- Witte B, Wolf M, Hillebrand H, et al. Extended cervical mediastinoscopy revisited. Eur J Cardiothorac Surg 2014;45:114-9. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res 2013;14:50. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Rochau U, Siebert U, et al. Cost-effectiveness of mediastinal lymph node staging in non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:1567-78. [Crossref] [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. [Crossref] [PubMed]
- Tournoy KG, Burgers SA, Annema JT, et al. Transesophageal endoscopic ultrasound with fine needle aspiration in the preoperative staging of malignant pleural mesothelioma. Clin Cancer Res 2008;14:6259-63. [Crossref] [PubMed]
- Beebe-Dimmer JL, Fryzek JP, Yee CL, et al. Mesothelioma in the United States: a Surveillance, Epidemiology, and End Results (SEER)-Medicare investigation of treatment patterns and overall survival. Clin Epidemiol 2016;8:743-50. [Crossref] [PubMed]


