
From minimal invasive extracorporeal circulation type I to type III: the perfusionist’s view
Introduction
The main challenge of modern cardiac perfusion technologies is the achievement of optimal biocompatibility for extracorporeal circulation (ECC) circuits. The unfavorable pathophysiological side effects of conventional cardiopulmonary bypass (CPB) circuits on the organ systems are triggered by complement system activation through foreign surfaces, hemodilution due to the priming volume, blood-air contact as well as negative and positive pressures in the reservoir (1,2). To overcome these effects, the concept of minimal invasive extracorporeal circulation (MiECC) circuits has evolved over the last 20 years as an alternative to the more conventional ECC circuits but also as an alternative to off-pump strategy for coronary artery bypass grafting (CABG) (3,4). The use of MiECC circuits is now expanding. These systems offer several potential advantages because they reduce the systemic inflammatory response and subsequent organ dysfunction (5,6). In order to be strongly characterized as MiECC, the main components of the system must include a closed CPB circuit; biologically inert blood contact surfaces; reduced priming volume; a cardioplegia system; a venous bubble trap/venous air removing device and a shed blood management system (6). MiECC circuits are classified in four different types with modular components, which are described in the following sections.
MiECC type I and II
The main goal of type I is the avoidance of CPB related shortcomings. The MiECC type I consists of a closed circuit, which includes the oxygenator and the pump. The circuit has no open venous reservoir. All components of the MiECC circuits are coated with heparin and the tubing system is significantly reduced in length. The main applications of type I is extracorporeal life support (ECLS) and CABG. For CABG, that is a closed heart procedure, it is possible to reduce the MiECC systems to a minimum (6), i.e., to the following components: a centrifugal pump; a membrane oxygenator and tubing lines. A venous bubble trap is not incorporated. Furthermore, no venting lines are included and the removal as well as retransfusion of shed blood is only possible with cardiotomy suction. The latter is the main difference between type I and type II. The MiECC circuits type II includes a venous bubble trap or a venous air-removing device. Additionally, a pulmonary artery vent can be integrated in the MiECC type II circuit. Furthermore, shed blood can be separated and processed in both type I and type II circuits with cardiotomy suction (3). Due to the closed circuits construction of the MiECC there is no shifting of volume and the patient’s own venous capacitance serves as a volume compensation system. To avoid a volume overload with consecutive hemodilution the cardioplegia strategy needs to adapt. Therefore, the integration of low-volume cardioplegia technique is ideal and feasible in MiECC concepts (7). All components of the MiECC circuits are coated with heparin and the tubing system is significantly reduced in length. These characteristics permit for a reduction of the priming volume of between 500 and 650 mL compared to the standard ECC (3,8). The reduction of foreign surface area, separation of shed blood and the avoidance of blood-air contact also allow some reduction of the activated clotting time (ACT) targets in type I and type II MiECC circuits (3,8). Anticoagulation is achieved by administration of heparin infusion at 150 IE/kg with an ACT target of 300–500 seconds (6,9).
From type I/II to type III
For open heart procedures, e.g., aortic and mitral valve surgery, the physiology of ECC changes fundamentally. In valvular surgery, where “venting” is routinely used and blood-air interaction is much more present, the modular set-up of the MiECC circuits need to be adapted to such procedures (10). The main difference between MiECC type I/II und MiECC type III is the ability to control volume shifts and variations more efficiently. This is achieved by different modular setups: First, the integration of a volume collection bag into the MiECC circuit, for situations during which simple patient positioning manoeuvres are insufficient to correct in excessive volumes shifts (10). Secondly, the implementation of specific components, such as an additional bubble trap, soft bag or a hard-shell reservoir to handle collected vent blood.
The main challenge for the MiECC type III is to preserve the main principles of the minimal invasive extracorporeal technology (MiECT), such as: a closed ECC circuit; biologically inert blood contact surfaces; reduced priming volume; a cardioplegia system; a venous bubble trap/venous air removing device and a shed blood management system (6), while maintaining the equilibrium and the ability to manage excessive volume in a closed extracorporeal circuit.
However, one of the main issues for MiECC type III is the integration of one or more following components: (I) pulmonary artery vent; (II) aortic root vent; (III) left ventricular (LV) venting. Those modifications will help to guarantee a blood free surgical field.
Pulmonary artery venting: The main advantage is the possibility to connect the vent directly to the venous MiECC line. Therefore, the vent performance is directly depending on the negative pressure generated by the centrifugal pump. However, this is also the limiting factor for vent performance. Thus, the speed of the centrifugal pumps needs to be adjusted to the amount of the venous return in order to avoid venous vascular collapse and a subsequent instability of the perfusion circuit. Furthermore, pulmonary artery venting does not directly drain the left ventricle and cannot actively de-air the left heart during the reperfusion phase.
The retransfusion of the volume drained by pulmonary artery vent into the MiECC circuit without any blood-air contact is one of the major advantages of this approach and can be performed safely without any further components.
LV venting: the integration of a LV vent combines the advantages of better LV drainage and effective de-airing during the reperfusion phase. However, one of the principles of MiECT is given up: i.e., the avoidance of blood-air contact.
Consequently, the shed blood collected by the LV vent cannot be drained directly into the MiECC circuit without risking dangerous air entry. There are different approaches for retransfusion of the blood volume vented from the LV. One possibility is the use of a venous bubble trap which is connected in line with a soft bag reservoir for direct retransfusion into the MiECC circuit.
Another option, which avoids using a venous bubble trap, is the direct but manual cross-clamped connection to a hard-shell reservoir (Figure 1). The collected volume can be intermittently retransfused into the MiECC circuit by manually de-clamping the connection line. Also, the LV vent and pulmonary artery vent can both be controlled by an additional roller pump and therefore remain independent of the performance of the centrifugal pump.
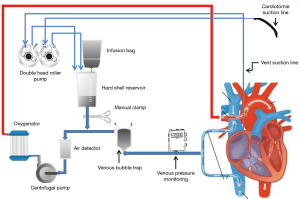
This enables an improved performance control and avoids venous drainage-induced perfusion collapse.
Summary
The flow performance of MiECC circuits type I and II is directly dependent on the venous return, with the patient’s venous capacitance serving as a volume compensation system. Both types cannot adapt to large volume shifts and thus have a limited range of performance. The MiECC circuits type I and II are therefore primarily used in closed-heart surgeries (e.g., CABG procedures) and for ECLS. Both circuit types offer effective reduction of system components and fulfil all principles of MiECC. The reduction of foreign surface area, separation of shed blood and the avoidance of blood-air contact even permit for a reduction of the ACT targets in type I and type II MiECC circuits (3,8). Cardiac surgery using MiECC type I and II requires intensive communication between the perfusionist, cardiac surgeon and the anaesthesiologist.
For open heart surgery, e.g., aortic and/or mitral valve surgery, the MiECC type III is able to control the volume shift more efficiently through the implementation of a volume reservoir bag and of either pulmonary artery venting or LV venting component (10). One of the main requirements during use of MiECC type III is the retransfusion of shed blood. The primary goal in the handling of shed blood in MiECC is its separation by using autologous retransfusion systems (3,11) Very critically thinking, types III and IV diverge from the very original concept of minimized CPB circuit.
To this regard, evidence is lacking as to whether the use of intraoperative autologous retransfusion systems reduces allogenic blood product utilization and which amount of shed blood volume can be processed without negatively influencing non-surgical haemostasis (3,11).
The requirement for MiECC types I and II focus mainly on maintaining a sufficient circulation during CABG procedures and ECLS. In contrast, the requirements for MiECC type III are higher since preservation of volume equilibrium is mandatory by closely managing shed blood retransfusion and avoiding venous collapse through close observation of centrifugal pump speed. Furthermore, if a hard-shell reservoir is used for LV venting or shed blood collection, the blood-air contact cannot be avoided completely, which in turn will increase inflammatory response (12).
The classification of MiECC circuits provides a clear definition of the different systems, and also give a clear distinction between conventional ECC und MiECC. MiECC presents a physiologically based perfusion strategy, not just another CPB circuit or a particular product. For this reason, a multidisciplinary approach is mandatory. Close collaboration between surgeons, anaesthesiologists and perfusionists is of paramount importance for the safe and efficient application of MiECC concepts (6).
Acknowledgments
The authors would like to thank Martin Schrag, Etienne Zermatten, Jolanda Consiglio and Anne-Kathrin Beese for their help and assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Edmunds LH Jr. Blood-surface interactions during cardiopulmonary bypass. J Card Surg 1993;8:404-10. [Crossref] [PubMed]
- Edmunds LH Jr. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 1998;66:S12-6; discussion S25-8.
- Jenni H, Rheinberger J, Czerny M, et al. Autotransfusion system or integrated automatic suction device in minimized extracorporeal circulation: influence on coagulation and inflammatory response. Eur J Cardiothorac Surg 2011;39:e139-43. [Crossref] [PubMed]
- Anastasiadis K, Bauer A, Antonitsis P, et al. Minimal invasive Extra-Corporeal Circulation (MiECC): a revolutionary evolution in perfusion. Interact Cardiovasc Thorac Surg 2014;19:541-2. [Crossref] [PubMed]
- Mazzei V, Nasso G, Salamone G, et al. Prospective randomized comparison of coronary bypass grafting with minimal extracorporeal circulation system (MECC) versus off-pump coronary surgery. Circulation 2007;116:1761-7. [Crossref] [PubMed]
- Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg. 2016;22:647-62. [Crossref] [PubMed]
- Kairet K, Deen J, Vernieuwe L, et al. Cardioplexol, a new cardioplegic solution for elective CABG. J Cardiothorac Surg 2013;8:120. [Crossref]
- Immer FF, Ackermann A, Gygax E, et al. Minimal extracorporeal circulation is a promising technique for coronary artery bypass grafting. Ann Thorac Surg 2007;84:1515-20; discussion 1521. [Crossref] [PubMed]
- Bauer A, Hausmann H, Schaarschmidt J, et al. Is 300 Seconds ACT Safe and Efficient during MiECC Procedures? Thorac Cardiovasc Surg 2019;67:191-202. [Crossref] [PubMed]
- Starinieri P, Declercq PE, Robic B, et al. A comparison between minimized extracorporeal circuits and conventional extracorporeal circuits in patients undergoing aortic valve surgery: is ‘minimally invasive extracorporeal circulation’ just low prime or closed loop perfusion ?. Perfusion 2017;32:403-8. [Crossref] [PubMed]
- Wang G, Bainbridge D, Martin J, et al. The efficacy of an intraoperative cell saver during cardiac surgery: a meta-analysis of randomized trials. Anesth Analg 2009;109:320-30. [Crossref] [PubMed]
- Westerberg M, Bengtsson A, Jeppsson A. Coronary surgery without cardiotomy suction and autotransfusion reduces the postoperative systemic inflammatory response. Ann Thorac Surg 2004;78:54-9. [Crossref] [PubMed]


