
Multiple primary lung cancer: a rising challenge
With the use of high-resolution chest imaging system and lung cancer screening program, patients with multiple primary lung cancers (MPLCs) are becoming a growing population in clinical practice worldwide (1-3). The crucial issue regarding multiple lung cancers is whether they should be diagnosed and treated as separate primary lesions or metastasis, on which both the stage classification and the planning of subsequent treatments are based (3,4). Histological differences between the multiple tumors are reliable indicators of MPLCs, but it would be rather challenging to differentiate a second primary cancer from a satellite, metastatic, or recurrent lesion of the original tumor if the multiple tumors are histologically same or similar. According to the 8th TNM staging system, the patients should be staged as T3 with additional tumor(s) within the same lobe; T4 with an ipsilateral lesion in a separate lobe, and M1a with a contralateral tumor in a separate lobe (5). However, this staging system could probably cause inaccurate assessment and treatment of patients with actual MPLCs, who are considered to have a local disease and may benefit the most from surgical resections (3,6).
Currently there are no unambiguous guidelines available for the diagnosis and treatment of MPLCs. Although there are some cases reported to The Surveillance, Epidemiology, and End Results (SEER), National Cancer Database (NCDB), and databases in Europe and Asia and recommendations for the management of MPLCs have been published by three major lung cancer research institutes [Union for Inter-national Cancer Control (UICC), American Joint Committee on Cancer (AJCC), and International Association for the Study of Lung Cancer (IASLC)], controversies still exist due to inter-individual varieties among patients. The primary objective of our review is to get a global understanding of current information and, taking various clinicopathological and genetic features into consideration, present diagnosis, classification, and multidisciplinary management strategies in patients with MPLCs. We will also make an effort to illuminate the rising challenge faced by physicians and surgeons worldwide regarding the optimal strategies of diagnoses and management of patients with MPLCs and try to draw some useful conclusion.
The definition and classification of MPLCs
Clinicopathological criteria
The clinical and pathological criteria for the diagnosis of MPLCs were first established by Martini and Melamed (7) in 1975 and then revised by Antakli and colleagues (8). The American College of Chest Physicians (ACCP) developed the criteria of diagnose in the year of 2007 with further clinical evaluations including lymphatic spread and systemic metastasis and extended the interval between metachronous MPLC as at least 4 years (9,10).
According to Martini-Melamed, a second tumor with different histological type from that of the primary one meets the criteria to be diagnosed as metachronous multiple primary lung cancer (mMPLC) .When identical or similar histology occurs, at least one of the following circumstances should be satisfied to differentially diagnose a new primary cancer from recurrence: at least a 2-year disease-free interval between the two tumors; development of the new lesion from an in situ carcinoma, or existence of the second tumor in another lobe or the other lung; ruling out extra pulmonary metastases and lymphatic spread common in both tumors (7). Antakli and colleagues then proposed a revised set of criteria (8), which were further extended by the ACCP guidelines (11), elongating the disease-free interval up to 4 years (Table 1).

Full table
Coexisting primary lung cancers are called synchronous multiple primary lung cancer (sMPLC). Based on Martini-Melamed criteria, the coexisting tumors should be physically separate and can present either same or different histology. When histology is identical or similar, tumors located in different segments, lobes, or lungs should originate from carcinomas in situ and, at the time of diagnosis, evidence of systematic metastases or lymphatic spread should be excluded (12) (Table 1).
Although the diagnostic criteria have been greatly improved, the diagnosis and classification of MPLCs still has not reached consensus amongst UICC, IASLC and AJCC (13). The 2012 UICC manual suggests that a tumor in the same organ with different histology should be diagnosed as a new tumor while IASLC suggests that the maximum T category and staging should be assigned and in addition the number of tumors should be mentioned (14,15). The combination of all tumors with consistent TNM designation should be used when staging sMPLC. The IASLC guide-line suggests that the TNM staging system can be functional in both same and different histology between primary and secondary tumors (9,14). The diagnosis and classification and therefore the planning of treatment are still difficult clinical decisions for physicians and surgeons due to the lack of high-level, evidence-based studies.
Molecular biomarkers
To better define the relationship among multiple lesions in lung, alternative approaches using novel molecular testing, such as immunohistochemical and molecular analysis, have been proposed by more recent studies (12,16). The clonality of multiple lesions can be demonstrated based on the array comparative genomic hybridization analysis, the loss of heterozygosity (LOH) analysis or the occurrence of somatic mutations in tumor suppressor genes or oncogenes (Table 2).

Full table
LOH, the loss of one allele at a specific locus caused by a deletion mutation or loss of a chromosome from a chromosome pare resulting in abnormal hemizygosity, represents 1 of the 2 hits required for tumor suppressor gene inactivation (43,44). LOH analysis is based on the comparing between tumor DNA and matched control DNA obtained from normal tissues or between single nucleotide polymorphism or microsatellite genotypes (27,45). Huang and colleagues reported that with the use of LOH array, the metachronous and synchronous primary lung tumors with identical histology could be successfully distinguished from intrapulmonary metastases (22). Shimizu and colleagues reported that the use of the combination of LOH and p53 mutation analysis could significantly increase the sensitivity and specificity in the identification of MPLCs (46). Despite the significant variability in genetic profiles among multiple primary lesions, some studies observed higher rates of LOH pattern accordance among multiple lesions (27).
Array-based comparative genomic hybridization (aCGH), mainly focused on the identification of copy number changes throughout the genome, is a robust method for undertaking comprehensive genomic level researches (47,48). With very high confidence rates, it is an attractive method that can be used to distinguish recurrent lesions from multiple tumors (49). Arai and colleagues reported that the use of aCGH assessment could improve the accuracy of the clinicopathological diagnosis of MPLCs (4). Similar conclusions could be found in Girard’s study (25). However, Girard and colleagues has also demonstrated that aCGH is costly, time consuming and requires relatively large amount of sample DNA, which is disadvantageous to use aCGH in clinical practices (25,26). It is also more practical to use aCGH in synchronous tumors since fresh frozen tissue is a crucial requirement (12).
Ideal genetic biomarkers for clonality analysis should be independent with frequent somatic mutations, which occurred early and maintained across the development of tumor. With rates up to 50% in non-small cell lung cancers (NSCLC), the p53 gene mutations are frequently seen in the presence of lung carcinomas (50,51). Most of them are point mutations and distribute throughout the DNA-binding domains of the p53 gene (51). Therefore, a huge amount of somatic mutations may occur and the chance of two independent lung cancers harboring identical mutations simultaneously is small. Recent studies demonstrated that p53 mutation analysis could be an effective biomarker for a definitive diagnosis in almost 66% of synchronous and metachronous cancer cases (23,52,53). Kirsten rat sarcoma viral oncogene homolog (K-ras) gene and epidermal growth factor receptor (EGFR) mutations are early events in the occurrence of lung cancer (54,55). Takamochi and colleagues reported that both K-ras and EGFR mutations frequently occur randomly in multifocal lung adenocarcinomas. Combined mutation pattern analyses of EGFR and K-ras may be useful for diagnoses and classification of MPLCs and therefore making decisions regarding treatment strategies (30). Chang and colleagues demonstrated that EGFR mutation, either alone or together with p53, is a potential biomarker for the clonal origin of MPLCs for differential diagnosis, especially in cases with similar histopathological types (24).
Despite that higher diagnostic rates (up to 83%) of using gene mutation pattern analysis in MPLCs have been described in several studies, it has also been reported that there is significant variability in genetic profiles among metachronous and synchronous primary tumors. Kalikaki and colleagues’ study suggested that multiple lesions may have matched mutations while metastasis may have additional mutations (56). It strongly indicated the possibility that subclonal drifts cause monoclonal origin with subsequent genetic tumor heterogeneity. Zhang and colleagues investigated the intra-tumor heterogeneity in 11 lung cancer patients and by multi-region whole-exome sequencing, all tumors showed clear evidence of ITH (57). Chang and Kalikaki’s studies demonstrated that p53 and EGFR mutation/overexpression status were distinctive between primary tumors and lymphatic spread in patients with NSCLC (56,58). Other studies also reported intra-tumoral heterogeneity of the EGFR mutation in NSCLC (59-61). It strongly indicated that the variations and complicates in the definition of synchronous and metachronous primary lung cancers by using molecular biomarkers (2).
Multidisciplinary management of MPLCs
The diagnosis and stage classification of MPLCs
Due to tumor heterogeneity and insufficient understanding of clinicopathological characteristics of MPLCs, there are currently no golden diagnostic criteria for MPLCs. The ACCP recommended that the diagnosis of MPLCs should be based on a careful review that considers all available information by a multidisciplinary tumor board, which should include radiologist, thoracic surgeons, pathologists and pulmonologists (9-11,62).
Stage classification of multiple lesions is crucial for the surgical treatment because it allows consistent diagnosis of patients (63). For patients with MPLCs, however, the staging rules are ambiguous and confusing. Previous studies demonstrated that, for sMPLC, each tumor should be staged and treated as separate tumors and one TNM designation should be assigned in the final stage based on a combination of all tumors. For mMPLC, the second tumor should be staged as a primary original tumor (11,64).
According to ACCP guidelines (11,65), for patients with multiple primary NSCLCs (synchronous or metachronous), when therapeutic surgical resection is considered, invasive LN biopsy (if possible) and extra-thoracic imaging (head CT/magnetic resonance imaging plus whole-body PET or abdominal CT plus bone scan) are recommended (grade 1B). And the possibility of a benign lesion or synchronous primary lung cancer should be considered and excluded in patients with suspected or diagnosed lung cancer and an ipsilateral different lobe nodule (grade 1C). Preoperative bronchoscopy examination could also be beneficial for the evaluation of local tumor extension and surgical treatment. The size and location of the tumors and the patient’s general condition should be both carefully evaluated before choosing a surgical approach.
Surgical treatment: lobectomy or sublobectomy?
Despite that surgical resection remains the most employed approach for the treatment of MPLCs, controversies over some issues still exist. Promising survival outcomes of lobectomy have been demonstrated in previous studies (66,67), however, standard surgical strategies for the treatment of MPLCs have not been established because of the lack of consistent golden diagnoses criteria and prospective clinical trials. The extent of resection is mainly decided by surgeons based on the balance of risk and benefit of surgery, taking characteristics of the tumor and status of patients into consideration, which might have inter-individual differences (16,68,69) (Table 3).
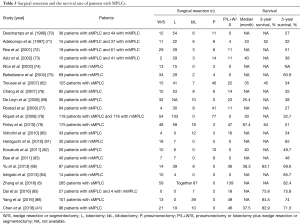
Full table
For mMPLC, anatomical removal of the second lesion with lobectomy or bilobectomy was the first choice for surgery in the majority of the previous reports (64,87). However, in other studies, sublobar resections, including segmentectomy or wedge resection, were the mainstream of treatment (86). For sMPLC, which more frequently occurred in the same lung, anatomical resections (single, bilobectomy, or pneumonectomy) are also recommended (85). Chang et al. demonstrated that anatomical resection of the first lesion followed by limited resection of the second might be a safer and more beneficial option for synchronous bilateral lesions (73,76,77,80). The initial surgery should be performed on the side with the largest tumor and contralateral resection should then follow, according to recommendation (79). Nakata and colleagues reported bilateral video-assisted thoracic surgery in surgical treatment of cases with bilateral MPLCs might come alone with minor postoperative complications and good survival rate also (88). These studies indicated that in patients who are intolerant to an extensive surgical resection, the sublobectomy has been widely accepted as an alternative choice since it allows major conservation of lung function (16). However, it still needs to be addressed that the local recurrence rate after sublobectomy (wedge or segmental resection) is higher than that after lobectomy (46,89,90).
Surgical treatment or stereotactic body radiation therapy (SBRT)?
A certain amount of patients with MPLCs might be intolerant to surgical resections due to impaired cardiopulmonary function or other impaired conditions. SBRT, also called stereotactic ablative radiotherapy (SABR), is a novel radiation modality that has been recommended as an optional therapeutic strategy for patients (91-93). For patients with early stage NSCLC, several groups have reported similar outcomes after SBRT treatment and surgical resections (93-95). For the patients with NSCLCs but intolerant to surgeries, Timmerman and Nantavithya’s studies demonstrated the feasibility, safety, and efficacy of this technique (91,96). The local control rate after SBRT is more than 90% (97,98). Moreover, SBRT has also been widely adopted for the treatment of oligometastasis involving the lungs (87,99).
Chang and colleagues reported that the 2- and 4-year local control rates of SBRT treating MPLCs were 97.4% and 95.7%. The 2- and 4-year overall survival (OS) were 73.2% and 47.5% and progression-free survival (PFS) were 67.0% and 58.0%. Patients with sMPLC had lower OS and PFS than patients with mMPLC (100). Creach and colleagues investigated MPLCs patients in whom SBRT was used for at least one tumor. The 2-year OS was acceptable and no grade ≥3 toxicities were observed (101), which were similar to Matthiesen’s reports (102). Varlotto and colleagues described the OS, recurrence rate, and loco-regional control rate of SBRT treatment were acceptable compared with those observed after surgical treatment (103). However, in these studies, most of the patients with MPLCs submitted to SBRT treatment were those who are intolerant to anatomic resection.
It is reported that the regional and overall recurrence rates after SBRT for single early stage lung cancer were up to 10% and 30%, respectively (104). One of the major disadvantages of SBRT without surgery treatment is that exact pathologic mediastinal lymph node histology thus the accurate staging is unavailable. Although PET/CT has quite considerable specificity and sensitivity in the detection of lymph node spread, the false-negative rate is still 13% for NSCLC patients in clinical stage T1-2N0 (105). Takashi and colleagues reported that the regional recurrence rate of patients with MPLCs was about twice as much as that of patients with single early stage lung cancer, indicating the risk of regional node micrometastasis of each lesion and the potential application of adjuvant chemotherapy for eradication of micrometastasis (106). These studies indicated that the clinical application of SBRT in treating MPLCs still needs further research and observation.
Targeted therapy: is there a role for it?
Another potential treatment for the medically inoperable patients with MPLCs is targeted therapy, especially the EGFR-targeted tyrosine kinase inhibitors. It has been widely reported that the NSCLC patients carrying activating mutations in EGFR might respond to EGFR-targeted tyrosine kinase inhibitors (107,108). Nevertheless, as mentioned previously, most of the lesions from MPLCs are histopathologically different or have different molecular alternations. Thus, the gene mutation testing results from biopsied or resected lesions might not fully represented the genetic changes of all the lesions in the lung, which may greatly limit the use of target therapy in the management of MPLCs.
Ye and colleagues reported a successful case of treatment of a sMPLC patient displaying heterogeneous EGFR and K-ras molecular profiles and different responses to gefitinib. Not feasible for aggressive surgical resection, the patient was initially treated with gefitinib. Considering the different responses among the multiple lesions, a strategy involving continuing gefitinib treatment for the gefitinib-sensitive bilateral ground-glass opacity (GGO) lesions and surgical resection for the gefitinib insensitive lesion was developed. The patient achieved complete remission and has been free of disease for 1.5 years (109). Their study indicated that the potential role of target therapy in the multidisciplinary management of MPLCs. However, in a study involving 78 patients with multifocal adenocarcinomas presenting as GGO lesions, Liu and colleagues found that matched EGFR mutations between the primary lesions and the paired specimen collected from another lesion were identified in only 8% of the patients (36). Ryoo and colleagues’ study also showed that the multiple lesions response to EGFR-tyrosine kinase inhibitors might be caused by different molecular pathogenesis (110). Given the fact that EGFR-targeted tyrosine kinase inhibitors do not restrain growth of tumors without EGFR mutations, these results indicated that the genetic heterogeneity of multifocal adenocarcinomas may bring a challenge to get a whole control of disease in all cancer sites (2).
Multiple GGOs
One issue worthy to be specially addressed is the multiple ground-glass opacities (GGOs). GGOs, mainly including pure GGO (pGGO) and part-solid GGO, often characterized as a focal area in lung with increased attenuation on CT scan through which normal parenchymal structures can still be visualized (111). Lung cancers growing in a lepidic pattern can present as a GGO because the tumor cells grow only along the alveoli, therefore allowing aeration of the alveoli (2,36). Several studies documented that GGOs often represent benign lesion, or relative lower grade malignant lesions, such as atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), or minimally invasive adenocarcinoma (MIA). The presence of a solid component in GGO, however, strongly suggests the presence of cancer invasion (111,112). The Fleischer Society believed multiple GGOs should be considered as MPLCs rather than intrapulmonary metastasis which was concordant with IASLC (113), but evidence to reliably identify multiple GGO lesions as clonal or otherwise remains unclear.
Unlike solid nodules, the progression of GGOs is usually very slow. Hiramatsu and colleagues demonstrated that initial size of GGO and a history of lung cancer were independent factors that were significantly associated with GGO growth during follow-up. Their data showed that the growth rate at 5 years was 66% in the GGO lesions with diameters larger than 10 mm (114). Tsutsui and colleagues demonstrated that most of the pGGO and part-solid GGO are clinically stable, only about 20% of them would decrease, disappear, or advance (115). Additional data showed that worse prognosis was associated with the larger size of the solid component of a lesion. Kim and colleagues demonstrated that for a pGGO lesion larger than 8 mm, resection should be performed to rule out the possibility of malignancy, whereas for a pGGO less than 8 mm, closely followed up using imaging studies is strongly recommended (112).
To our best knowledge, only a few of studies investigated the characteristics of coexisting GGOs and solid nodules. Therefore, clinical controversy over treatment strategies for multiple GGOs still exists. Godoy and Naidich demonstrated that surgical resection could be considered for mixed GGOs, while solitary pure GGO should be followed up until they increase in size or develop new solid component (113,116). Chen and colleagues evaluated both clinical characteristics and genetic alterations of pure GGO, part-solid nodules, and solid-dominant nodules. The 5-year recurrence-free survival was 100% in patients with multiple GGOs, 68% in those with one solid lesion, and 51.4% in those with two solid nodules. The 5-year overall survival was 100% in pure GGO, 80.5% in part-solid nodule, and 59.9% in solid-dominant nodule. A high rate of variability of genetic alterations (89.7%) was observed between cancers within individual patients (41). Similar results were presented in Gu and Castiglioni’s studies, which reported the high frequency of genetic heterogeneity among multiple lesions in the same patient (117,118). These studies indicated that, compared to multiple solid NSCLC, the multiple GGOs have their unique clinicopathological characteristics and genetic features. The further explorations should be focused on the potential need to perform collaborative molecular tests in patients with multiple lesions, the possible role of EGFR mutation in staging of multiple GGOs and the indication of EGFR inhibitors for patients with multifocal adenocarcinomas presenting as GGOs (2,36).
Rising challenge
The growing population of patients with MPLCs is now a rising challenge for physicians and surgeons worldwide. The SEER, NCDB and some other database have been collecting cases of MPLCs but due to the ambiguous criteria for diagnosis and guidelines for the treatment, more effort should be emphasized on it. We summarized the studies now available worldwide (Tables 2,3) and found that molecular biomarkers are playing important roles in the diagnosis of MPLCs and more and more biomarkers would be discovered to make better diagnostic accuracy. Surgical treatment is still the optimal choice for the patients with MPLCs, but it can be a dilemma in some cases and more experience and data analyses will help the surgeons to make better choices. Also, the SBRT and targeted therapy are also innovative and potentially efficient treatment methods which require further research.
Conclusions
With special clinicopathological characteristics and genetic features, MPLCs are increasingly encountered in clinical practice. Comprehensive molecular analysis could be helpful in differentiating multiple primary tumors from metastases. Although surgical resection remains the mainly choice for the treatment of MPLCs, the target therapy and SBRT may have their own roles in the multidisciplinary management of MPLCs. Nevertheless, there are still several controversies exist in the diagnosis, classification, and multidisciplinary management strategies of MPLCs. Multiple GGOs are unique MPLCs that need special attentions in the clinical practice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vazquez M, Carter D, Brambilla E, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009;64:148-54. [Crossref] [PubMed]
- Tanvetyanon T, Boyle TA. Clinical implications of genetic heterogeneity in multifocal pulmonary adenocarcinomas. J Thorac Dis 2016;8:E1734-8. [Crossref] [PubMed]
- Asamura H. Multiple primary cancers or multiple metastases, that is the question. J Thorac Oncol 2010;5:930-1. [Crossref] [PubMed]
- Arai J, Tsuchiya T, Oikawa M, et al. Clinical and molecular analysis of synchronous double lung cancers. Lung Cancer 2012;77:281-7. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Zhang Z, Gao S, Mao Y, et al. Surgical Outcomes of Synchronous Multiple Primary Non-Small Cell Lung Cancers. Sci Rep 2016;6:23252. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]
- Antakli T, Schaefer RF, Rutherford JE, et al. Second primary lung cancer. Ann Thorac Surg 1995;59:863-6; discussion 867. [Crossref] [PubMed]
- Shen KR, Meyers BF, Larner JM, et al. Special treatment issues in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:290S-305S.
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-e399S.
- Alberts WM; American College of Chest Physicians. Introduction: Diagnosis and management of lung cancer: ACCP evidence-based clinical practice guidelines (2nd Edition). Chest 2007;132:20S-22S.
- Loukeri AA, Kampolis CF, Ntokou A, et al. Metachronous and synchronous primary lung cancers: diagnostic aspects, surgical treatment, and prognosis. Clin Lung Cancer 2015;16:15-23. [Crossref] [PubMed]
- Fonseca A, Detterbeck FC. How many names for a rose: inconsistent classification of multiple foci of lung cancer due to ambiguous rules. Lung Cancer 2014;85:7-11. [Crossref] [PubMed]
- Jiang L, He J, Shi X, et al. Prognosis of synchronous and metachronous multiple primary lung cancers: systematic review and meta-analysis. Lung Cancer 2015;87:303-10. [Crossref] [PubMed]
- Giroux DJ, Rami-Porta R, Chansky K, et al. The IASLC Lung Cancer Staging Project: data elements for the prospective project. J Thorac Oncol 2009;4:679-83. [Crossref] [PubMed]
- Lin MW, Wu CT, Kuo SW, et al. Clinicopathology and genetic profile of synchronous multiple small adenocarcinomas: implication for surgical treatment of an uncommon lung malignancy. Ann Surg Oncol 2014;21:2555-62. [Crossref] [PubMed]
- Noguchi M, Maezawa N, Nakanishi Y, et al. Application of the p53 gene mutation pattern for differential diagnosis of primary versus metastatic lung carcinomas. Diagn Mol Pathol 1993;2:29-35. [Crossref] [PubMed]
- Sozzi G, Miozzo M, Pastorino U, et al. Genetic evidence for an independent origin of multiple preneoplastic and neoplastic lung lesions. Cancer Res 1995;55:135-40. [PubMed]
- Mitsudomi T, Yatabe Y, Koshikawa T, et al. Mutations of the P53 tumor suppressor gene as clonal marker for multiple primary lung cancers. J Thorac Cardiovasc Surg 1997;114:354-60. [Crossref] [PubMed]
- Hiroshima K, Toyozaki T, Kohno H, et al. Synchronous and metachronous lung carcinomas: molecular evidence for multicentricity. Pathol Int 1998;48:869-76. [Crossref] [PubMed]
- Matsuzoe D, Hideshima T, Ohshima K, et al. Discrimination of double primary lung cancer from intrapulmonary metastasis by p53 gene mutation. Br J Cancer 1999;79:1549-52. [Crossref] [PubMed]
- Huang J, Behrens C, Wistuba I, et al. Molecular analysis of synchronous and metachronous tumors of the lung: impact on management and prognosis. Ann Diagn Pathol 2001;5:321-9. [Crossref] [PubMed]
- van Rens MT, Eijken EJ, Elbers JR, et al. p53 mutation analysis for definite diagnosis of multiple primary lung carcinoma. Cancer 2002;94:188-96. [Crossref] [PubMed]
- Chang YL, Wu CT, Lin SC, et al. Clonality and prognostic implications of p53 and epidermal growth factor receptor somatic aberrations in multiple primary lung cancers. Clin Cancer Res 2007;13:52-8. [Crossref] [PubMed]
- Girard N, Ostrovnaya I, Lau C, et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res 2009;15:5184-90. [Crossref] [PubMed]
- Girard N, Deshpande C, Lau C, et al. Comprehensive histologic assessment helps to differentiate multiple lung primary nonsmall cell carcinomas from metastases. Am J Surg Pathol 2009;33:1752-64. [Crossref] [PubMed]
- Wang X, Wang M, MacLennan GT, et al. Evidence for common clonal origin of multifocal lung cancers. J Natl Cancer Inst 2009;101:560-70. [Crossref] [PubMed]
- Moffatt-Bruce SD, Ross P, Leon ME, et al. Comparative mutational profiling in the assessment of lung lesions: should it be the standard of care? Ann Thorac Surg 2010;90:388-96. [Crossref] [PubMed]
- Girard N, Lou E, Azzoli CG, et al. Analysis of genetic variants in never-smokers with lung cancer facilitated by an Internet-based blood collection protocol: a preliminary report. Clin Cancer Res 2010;16:755-63. [Crossref] [PubMed]
- Takamochi K, Oh S, Matsuoka J, et al. Clonality status of multifocal lung adenocarcinomas based on the mutation patterns of EGFR and K-ras. Lung Cancer 2012;75:313-20. [Crossref] [PubMed]
- Warth A, Macher-Goeppinger S, Muley T, et al. Clonality of multifocal nonsmall cell lung cancer: implications for staging and therapy. Eur Respir J 2012;39:1437-42. [Crossref] [PubMed]
- Lin MW, Wu CT, Shih JY, et al. Clinicopathologic characteristics and prognostic significance of EGFR and p53 mutations in surgically resected lung adenocarcinomas</=2 cm in maximal dimension. J Surg Oncol 2014;110:99-106. [Crossref] [PubMed]
- Zhang Y, Hu H, Wang R, et al. Synchronous non-small cell lung cancers: diagnostic yield can be improved by histologic and genetic methods. Ann Surg Oncol 2014;21:4369-74. [Crossref] [PubMed]
- Wu C, Zhao C, Yang Y, et al. High Discrepancy of Driver Mutations in Patients with NSCLC and Synchronous Multiple Lung Ground-Glass Nodules. J Thorac Oncol 2015;10:778-83. [Crossref] [PubMed]
- Zhou X, Tian L, Fan J, et al. Method for discriminating synchronous multiple lung cancers of the same histological type: miRNA expression analysis. Medicine (Baltimore) 2016;95:e4478. [Crossref] [PubMed]
- Liu M, He WX, Song N, et al. Discrepancy of epidermal growth factor receptor mutation in lung adenocarcinoma presenting as multiple ground-glass opacities. Eur J Cardiothorac Surg 2016;50:909-13. [Crossref] [PubMed]
- Schneider F, Derrick V, Davison JM, et al. Morphological and molecular approach to synchronous non-small cell lung carcinomas: impact on staging. Mod Pathol 2016;29:735-42. [Crossref] [PubMed]
- Yang Y, Yin W, He W, et al. Phenotype-genotype correlation in multiple primary lung cancer patients in China. Sci Rep 2016;6:36177. [Crossref] [PubMed]
- Asmar R, Sonett JR, Singh G, et al. Use of Oncogenic Driver Mutations in Staging of Multiple Primary Lung Carcinomas: A Single-Center Experience. J Thorac Oncol 2017;12:1524-35. [Crossref] [PubMed]
- Patel SB, Kadi W, Walts AE, et al. Next-Generation Sequencing: A Novel Approach to Distinguish Multifocal Primary Lung Adenocarcinomas from Intrapulmonary Metastases. J Mol Diagn 2017;19:870-80. [Crossref] [PubMed]
- Chen K, Chen W, Cai J, et al. Favorable prognosis and high discrepancy of genetic features in surgical patients with multiple primary lung cancers. J Thorac Cardiovasc Surg 2018;155:371-9.e1. [Crossref] [PubMed]
- Haratake N, Toyokawa G, Takada K, et al. Programmed Death-Ligand 1 Expression and EGFR Mutations in Multifocal Lung Cancer. Ann Thorac Surg 2018;105:448-54. [Crossref] [PubMed]
- Ogiwara H, Kohno T, Nakanishi H, et al. Unbalanced translocation, a major chromosome alteration causing loss of heterozygosity in human lung cancer. Oncogene 2008;27:4788-97. [Crossref] [PubMed]
- Tai AL, Mak W, Ng PK, et al. High-throughput loss-of-heterozygosity study of chromosome 3p in lung cancer using single-nucleotide polymorphism markers. Cancer Res 2006;66:4133-8. [Crossref] [PubMed]
- Mao X. Young BDLu YJ. The application of single nucleotide polymorphism microarrays in cancer research. Curr Genomics 2007;8:219-28. [Crossref] [PubMed]
- Shimizu S, Yatabe Y, Koshikawa T, et al. High frequency of clonally related tumors in cases of multiple synchronous lung cancers as revealed by molecular diagnosis. Clin Cancer Res 2000;6:3994-9. [PubMed]
- Fuhrmann C, Schmidt-Kittler O, Stoecklein NH, et al. High-resolution array comparative genomic hybridization of single micrometastatic tumor cells. Nucleic Acids Res 2008;36:e39. [Crossref] [PubMed]
- Cho EK, Tchinda J, Freeman JL, et al. Array-based comparative genomic hybridization and copy number variation in cancer research. Cytogenet Genome Res 2006;115:262-72. [Crossref] [PubMed]
- Wa CV, DeVries S, Chen YY, et al. Clinical application of array-based comparative genomic hybridization to define the relationship between multiple synchronous tumors. Mod Pathol 2005;18:591-7. [Crossref] [PubMed]
- Chang MY, Chong IW, Chen FM, et al. High frequency of frameshift mutation on p53 gene in Taiwanese with non small cell lung cancer. Cancer Lett 2005;222:195-204. [Crossref] [PubMed]
- Gibbons DL. Byers LAKurie JM. Smoking, p53 mutation, and lung cancer. Mol Cancer Res 2014;12:3-13. [Crossref] [PubMed]
- Kim HJ, Kim N, Choi YJ, et al. Clinicopathologic features of gastric cancer with synchronous and metachronous colorectal cancer in Korea: are microsatellite instability and p53 overexpression useful markers for predicting colorectal cancer in gastric cancer patients? Gastric Cancer 2016;19:798-807. [Crossref] [PubMed]
- Yalcinkaya U, Ozturk E, Ozgur T, et al. P53 expression in synchronous colorectal cancer. Saudi Med J 2008;29:826-31. [PubMed]
- Donahue JM. Role of p53 and EGFR as prognostic biomarkers in stage I non-small cell lung cancer. J Surg Oncol 2014;110:97-8. [Crossref] [PubMed]
- Yamaguchi F, Kugawa S, Tateno H, et al. Analysis of EGFR, KRAS and P53 mutations in lung cancer using cells in the curette lavage fluid obtained by bronchoscopy. Lung Cancer 2012;78:201-6. [Crossref] [PubMed]
- Kalikaki A, Koutsopoulos A, Trypaki M, et al. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer 2008;99:923-9. [Crossref] [PubMed]
- Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014;346:256-9. [Crossref] [PubMed]
- Chang YL, Wu CT, Shih JY, et al. Comparison of p53 and epidermal growth factor receptor gene status between primary tumors and lymph node metastases in non-small cell lung cancers. Ann Surg Oncol 2011;18:543-50. [Crossref] [PubMed]
- Gallegos Ruiz MI, van Cruijsen H, Smit EF, et al. Genetic heterogeneity in patients with multiple neoplastic lung lesions: a report of three cases. J Thorac Oncol 2007;2:12-21. [Crossref] [PubMed]
- Nakano H, Soda H, Takasu M, et al. Heterogeneity of epidermal growth factor receptor mutations within a mixed adenocarcinoma lung nodule. Lung Cancer 2008;60:136-40. [Crossref] [PubMed]
- Jiang SX, Yamashita K, Yamamoto M, et al. EGFR genetic heterogeneity of nonsmall cell lung cancers contributing to acquired gefitinib resistance. Int J Cancer 2008;123:2480-6. [Crossref] [PubMed]
- Trousse D, Barlesi F, Loundou A, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg 2007;133:1193-200. [Crossref] [PubMed]
- Lv J, Zhu D, Wang X, et al. The Value of Prognostic Factors for Survival in Synchronous Multifocal Lung Cancer: A Retrospective Analysis of 164 Patients. Ann Thorac Surg 2018;105:930-6. [Crossref] [PubMed]
- Xue X, Xue Q, Wang N, et al. Early clinical diagnosis of synchronous multiple primary lung cancer. Oncol Lett 2012;3:234-7. [Crossref] [PubMed]
- Alberts WM; American College of Chest Physicians. Diagnosis and management of lung cancer executive summary: ACCP evidence-based clinical practice guidelines (2nd Edition). Chest 2007;132:1S-19S.
- Yu YC, Hsu PK, Yeh YC, et al. Surgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers? Ann Thorac Surg 2013;96:1966-74. [Crossref] [PubMed]
- Li J, Yang X, Xia T, et al. Stage I synchronous multiple primary non-small cell lung cancer: CT findings and the effect of TNM staging with the 7th and 8th editions on prognosis. J Thorac Dis 2017;9:5335-44.
- Zuin A, Andriolo LG, Marulli G, et al. Is lobectomy really more effective than sublobar resection in the surgical treatment of second primary lung cancer? Eur J Cardiothorac Surg 2013;44:e120-5; discussion e5.
- De Leyn P, Moons J, Vansteenkiste J, et al. Survival after resection of synchronous bilateral lung cancer. Eur J Cardiothorac Surg 2008;34:1215-22. [Crossref] [PubMed]
- Deschamps C, Pairolero PC, Trastek VF, et al. Multiple primary lung cancers. Results of surgical treatment. J Thorac Cardiovasc Surg 1990;99:769-77; discussion 777-8. [PubMed]
- Adebonojo SA. Moritz DMDanby CA. The results of modern surgical therapy for multiple primary lung cancers. Chest 1997;112:693-701. [Crossref] [PubMed]
- Rea F, Zuin A, Callegaro D, et al. Surgical results for multiple primary lung cancers. Eur J Cardiothorac Surg 2001;20:489-95. [Crossref] [PubMed]
- Aziz TM, Saad RA, Glasser J, et al. The management of second primary lung cancers. A single centre experience in 15 years. Eur J Cardiothorac Surg 2002;21:527-33. [Crossref] [PubMed]
- Rice D, Kim HW, Sabichi A, et al. The risk of second primary tumors after resection of stage I nonsmall cell lung cancer. Ann Thorac Surg 2003;76:1001-7; discussion 7-8. [Crossref] [PubMed]
- Battafarano RJ, Force SD, Meyers BF, et al. Benefits of resection for metachronous lung cancer. J Thorac Cardiovasc Surg 2004;127:836-42. [Crossref] [PubMed]
- Chang YL. Wu CTLee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg 2007;134:630-7. [Crossref] [PubMed]
- Rostad H, Strand TE, Naalsund A, et al. Resected synchronous primary malignant lung tumors: a population-based study. Ann Thorac Surg 2008;85:204-9. [Crossref] [PubMed]
- Riquet M, Cazes A, Pfeuty K, et al. Multiple lung cancers prognosis: what about histology? Ann Thorac Surg 2008;86:921-6. [Crossref] [PubMed]
- Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205. [Crossref] [PubMed]
- Voltolini L, Rapicetta C, Luzzi L, et al. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. Eur J Cardiothorac Surg 2010;37:1198-204. [Crossref] [PubMed]
- Haraguchi S, Koizumi K, Hirata T, et al. Surgical treatment of metachronous nonsmall cell lung cancer. Ann Thorac Cardiovasc Surg 2010;16:319-25. [PubMed]
- Kocaturk CI, Gunluoglu MZ, Cansever L, et al. Survival and prognostic factors in surgically resected synchronous multiple primary lung cancers. Eur J Cardiothorac Surg 2011;39:160-6. [Crossref] [PubMed]
- Bae MK, Byun CS, Lee CY, et al. The role of surgical treatment in second primary lung cancer. Ann Thorac Surg 2011;92:256-62. [Crossref] [PubMed]
- Ishigaki T, Yoshimasu T, Oura S, et al. Surgical treatment for metachronous second primary lung cancer after radical resection of primary lung cancer. Ann Thorac Cardiovasc Surg 2013;19:341-4. [Crossref] [PubMed]
- Dai L, Yang HL, Yan WP, et al. The equivalent efficacy of multiple operations for multiple primary lung cancer and a single operation for single primary lung cancer. J Thorac Dis 2016;8:855-61. [Crossref] [PubMed]
- Yang H, Sun Y, Yao F, et al. Surgical Therapy for Bilateral Multiple Primary Lung Cancer. Ann Thorac Surg 2016;101:1145-52. [Crossref] [PubMed]
- Zhao H, Yang H, Han K, et al. Clinical outcomes of patients with metachronous second primary lung adenocarcinomas. Onco Targets Ther 2017;10:295-302. [Crossref] [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9. [Crossref] [PubMed]
- Mathisen DJ, Jensik RJ, Faber LP, et al. Survival following resection for second and third primary lung cancers. J Thorac Cardiovasc Surg 1984;88:502-10. [PubMed]
- Wainscoat JS, Fey MF. Assessment of clonality in human tumors: a review. Cancer Res 1990;50:1355-60. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Shinde A, Li R, Kim J, et al. Stereotactic body radiation therapy (SBRT) for early-stage lung cancer in the elderly. Semin Oncol 2018;45:210-9. [Crossref] [PubMed]
- Sebastian NT. Xu-Welliver MWilliams TM. Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC): contemporary insights and advances. J Thorac Dis 2018;10:S2451-64. [Crossref] [PubMed]
- Rusthoven CG. Jones BLKavanagh BD. Medical operability and inoperability drive survival in retrospective analyses comparing surgery and SBRT for early-stage lung cancer. J Thorac Cardiovasc Surg 2018;155:810-1. [Crossref] [PubMed]
- Donovan EK, Swaminath A. Stereotactic body radiation therapy (SBRT) in the management of non-small-cell lung cancer: Clinical impact and patient perspectives. Lung Cancer (Auckl) 2018;9:13-23. [Crossref] [PubMed]
- Nantavithya C, Gomez DR, Wei X, et al. Phase 2 Study of Stereotactic Body Radiation Therapy and Stereotactic Body Proton Therapy for High-Risk, Medically Inoperable, Early-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2018;101:558-63. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Louie AV, van Werkhoven E, Chen H, et al. Patient reported outcomes following stereotactic ablative radiotherapy or surgery for stage IA non-small-cell lung cancer: Results from the ROSEL multicenter randomized trial. Radiother Oncol 2015;117:44-8. [Crossref] [PubMed]
- Ogawa Y, Shibamoto Y, Hashizume C, et al. Repeat stereotactic body radiotherapy (SBRT) for local recurrence of non-small cell lung cancer and lung metastasis after first SBRT. Radiat Oncol 2018;13:136. [Crossref] [PubMed]
- Chang JY, Liu YH, Zhu Z, et al. Stereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancer. Cancer 2013;119:3402-10. [Crossref] [PubMed]
- Creach KM, Bradley JD, Mahasittiwat P, et al. Stereotactic body radiation therapy in the treatment of multiple primary lung cancers. Radiother Oncol 2012;104:19-22. [Crossref] [PubMed]
- Matthiesen C, Thompson JS, De La Fuente Herman T, et al. Use of stereotactic body radiation therapy for medically inoperable multiple primary lung cancer. J Med Imaging Radiat Oncol 2012;56:561-6. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Chi A, Liao Z, Nguyen NP, et al. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications. Radiother Oncol 2010;94:1-11. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Shintani T, Masago K, Takayama K, et al. Stereotactic Body Radiotherapy for Synchronous Primary Lung Cancer: Clinical Outcome of 18 Cases. Clin Lung Cancer 2015;16:e91-6. [Crossref] [PubMed]
- Lynch TJ. The evolving story of the epidermal growth factor receptor as a target for non-small-cell lung cancer. Clin Adv Hematol Oncol 2004;2:786-7. [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Ye C, Wang J, Li W, et al. Novel Strategy for Synchronous Multiple Primary Lung Cancer Displaying Unique Molecular Profiles. Ann Thorac Surg 2016;101:e45-7. [Crossref] [PubMed]
- Ryoo BY, Na II, Yang SH, et al. Synchronous multiple primary lung cancers with different response to gefitinib. Lung Cancer 2006;53:245-8. [Crossref] [PubMed]
- Dai C, Ren Y, Xie H, et al. Clinical and radiological features of synchronous pure ground-glass nodules observed along with operable non-small cell lung cancer. J Surg Oncol 2016;113:738-44. [Crossref] [PubMed]
- Kim HK, Choi YS, Kim K, et al. Management of ground-glass opacity lesions detected in patients with otherwise operable non-small cell lung cancer. J Thorac Oncol 2009;4:1242-6. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Hiramatsu M, Inagaki T, Inagaki T, et al. Pulmonary ground-glass opacity (GGO) lesions-large size and a history of lung cancer are risk factors for growth. J Thorac Oncol 2008;3:1245-50. [Crossref] [PubMed]
- Tsutsui S, Ashizawa K, Minami K, et al. Multiple focal pure ground-glass opacities on high-resolution CT images: Clinical significance in patients with lung cancer. AJR Am J Roentgenol 2010;195:W131-8. [Crossref] [PubMed]
- Godoy MC, Naidich DP. Overview and strategic management of subsolid pulmonary nodules. J Thorac Imaging 2012;27:240-8. [Crossref] [PubMed]
- Castiglioni M, Louie BE, Wilshire CL, et al. Patients with multiple nodules and a dominant lung adenocarcinoma have similar outcomes and survival compared with patients who have a solitary adenocarcinoma. Interact Cardiovasc Thorac Surg 2015;20:229-35. [Crossref] [PubMed]
- Gu B, Burt BM, Merritt RE, et al. A dominant adenocarcinoma with multifocal ground glass lesions does not behave as advanced disease. Ann Thorac Surg 2013;96:411-8. [Crossref] [PubMed]


