
Role of skeletal muscle on chest computed tomography for risk stratification of lung cancer patients
In his editorial “Sarcopenia is a potential factor for optimized treatment selection for elderly patients with early stage non-small-cell lung cancer recent retrospective analysis” Dr. Matsuo comments on our study “Thoracic Skeletal Muscle Is Associated With Adverse Outcomes After Lobectomy for Lung Cancer” (1). We appreciate the opportunity to reply to his comments and discuss how body composition metrics derived from routine computed tomography (CT) images of the thorax could improve lung cancer care.
First, we would like to emphasize that using muscle measurements for risk stratification does not necessarily equal sarcopenia assessment (2). As reported in Annals of Thoracic Surgery, we evaluated the association between thoracic skeletal muscle measured on chest CT examinations obtained prior to lobectomy and adverse outcomes in lung cancer patients (1). We chose to assess muscle mass at the level of the fifth thoracic vertebral body (T5) since this measurement captures some accessory respiratory muscles (1). While low muscle mass at T5 identified patients at risk for adverse outcomes we did not label these patients as sarcopenic (1). The latter is not yet possible in the absence of reference values for muscle mass at this level in a normal population (3). It should be noted, however, that such reference values recently became available for the level of the tenth thoracic vertebral body, albeit without adjustment for race (4).
Second, we share the sentiment that the evaluation of body composition on routinely acquired chest CT examinations is a tremendous opportunity to improve lung cancer care since this approach leverages existing information without subjecting patients to additional tests or ionizing radiation. As Dr. Matsuo points out, body composition analysis has been mainly studied at the level of the third lumbar vertebral body (L3). We showed in a recent study of thoracic muscle and overall survival in lung cancer patients undergoing pneumonectomy that chest CT examinations are obtained more frequently and closer to the time of surgery in these patients compared to CT of the abdomen and pelvis (5).
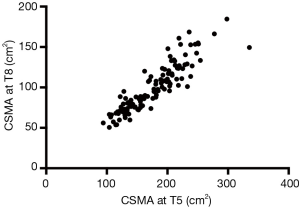
Third, we would like to emphasize that not all CT examinations obtained for clinical care are equal. As a matter of fact, image acquisition parameters such as field of view, intravenous contrast and radiation dose can significantly affect muscle segmentation (6). In that regard a detailed understanding of the technical aspects involved in image acquisition and image processing is essential to prevent segmentation errors. Patient positioning is part of the image acquisition process and we concur with Dr. Matsuo that cross-sectional muscle area in the upper thorax varies depending on arm positioning (i.e., arms above the head versus arms by the side), as previously documented by others (7). However, we did account for this aspect in our study by only including chest CT examinations of patients that had their arms raised above their head. Patients that had their arms by their side were excluded due to “patient positioning” (1). In this context it is interesting to consider that while correlation between muscle cross sectional area at T5 and at the level of the eight thoracic vertebral body (T8) is excellent (Pearson’s r=0.905), the latter is barely affected by arm position (Figure 1) (5).
Fourth, we do not understand Dr. Matsuo’s concern about a statistical commentary accompanying our paper (8). In essence, this commentary explains in detail why our statistical approach to time-to-event analysis is valid (8). We therefore do not consider statistical analysis a weakness of our paper.
Lastly, we would like to expand on the conclusion drawn by Dr. Matsuo since low muscle mass also matters for quality of life. Documented associations with sarcopenia at L3 in non-surgical lung cancer patients include increased depression, fatigue and pain (9,10). Therefore, we wholeheartedly agree with Dr. Matsuo that it will be crucial to work towards a definition of sarcopenia on chest CT. Standardization will facilitate the multicenter studies required to validate our findings in robust patient cohorts with the goal to improve lung cancer care by harnessing the information contained in routine chest CT examinations.
Acknowledgements
The authors would like to acknowledge Sheila J. Knoll, RN for her valuable contributions.
Footnote
Conflicts of Interest: FJ Fintelmann receives a grant from the Society of Thoracic Radiology related to this topic. The other authors have no conflicts of interest to declare.
References
- Fintelmann FJ, Troschel FM, Mario J, et al. Thoracic Skeletal Muscle Is Associated With Adverse Outcomes After Lobectomy for Lung Cancer. Ann Thorac Surg 2018;105:1507-15. [Crossref] [PubMed]
- Parkin E, Renehan AG. Need to Distinguish the Term Sarcopenia From Risk Stratification Derived From Muscle Parameters. J Clin Oncol 2018;36:2128-9. [Crossref] [PubMed]
- Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755-63. [Crossref] [PubMed]
- Derstine BA, Holcombe SA, Ross BE, et al. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 2018;8:11369. [Crossref] [PubMed]
- Troschel FM, Kuklinski MW, Knoll SJ, et al. Preoperative thoracic muscle area on computed tomography predicts long-term survival following pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Fuchs G, Chretien YR, Mario J, et al. Quantifying the effect of slice thickness, intravenous contrast and tube current on muscle segmentation: Implications for body composition analysis. Eur Radiol 2018;28:2455-63. [Crossref] [PubMed]
- Prakash P, Kalra MK, Digumarthy SR, et al. Alterations of anatomic relationships on chest computed tomography as a function of arm position. J Comput Assist Tomogr 2010;34:285-9. [Crossref] [PubMed]
- Lu E, Colditz GA. Statistical Commentary. Ann Thorac Surg 2018;105:1515. [Crossref] [PubMed]
- Nipp RD, Fuchs G, El-Jawahri A, et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist 2018;23:97-104. [Crossref] [PubMed]
- Bye A, Sjøblom B, Wentzel-Larsen T, et al. Muscle mass and association to quality of life in non-small cell lung cancer patients. J Cachexia Sarcopenia Muscle 2017;8:759-67. [Crossref] [PubMed]


