
Perioperative systemic magnesium sulphate to minimize acute and chronic post-thoracotomy pain: a prospective observational study
Introduction
Lung cancer is the most common cancer worldwide and is a leading cause of death. Thousands of patients annually undergo surgical resection of the lung parenchyma (1). The severity of acute postoperative pain has been linked to the development of persistent postoperative pain (2).
Remifentanil is an ultra-short-acting opioid characterized by rapid recovery. It is reliable and widely used in clinical practice (3). Continuous application of remifentanil can induce tolerance and hyperalgesia, which may lead to analgesic overconsumption and high pain scores (4) postoperative.
Chronic post-thoracotomy pain (CPTP) following thoracic surgery is common. CPTP is defined by the International Association for the Study of Pain as pain that recurs or persists along a thoracotomy incision for at least two months following the surgical procedure (5). The prevalence is reported to be between 50% and 80% and is usually mild or moderate, but in 5% the pain is severe and disabling (2,6). CPTP consist of different types of pain. Usually, it is a burning, dysesthetic, and aching feeling, which displays many features of neuropathic pain (7,8). Neuropathic pain poorly responds to the use of opioids (9).
The etiology of CPTP is not fully understood, but many patients experiencing CPTP have neuropathic pain rather than nociceptive pain (10). In chronic pain states, an inappropriate focus can be made of pain intensity. Pain intensity is measured with a unidimensional tool like the numerical rating scale (NRS). To assess neuropathic pain a multidimensional tool like the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) is indispensable (11). The LANSS pain scale can distinguish patients with neuropathic pain from those with nociceptive pain (12).
CPTP might be one of the most challenging conditions confronting physicians. Different strategies have been described to reduce acute and CPTP: nonsteroidal anti-inflammatory drugs, parenteral opioids, epidural and paravertebral infusion of local anesthetics, intercostal and phrenic nerve blockades, and cryotherapy (13). The results were inconstant and no single strategy has shown to be effective in all patients.
Magnesium sulphate (MgSO4) has been shown to produce an antinociceptive effect on animal models of neuropathic and inflammatory pain (14). Furthermore, magnesium is a physiological antagonist of the N-methyl-D-aspartate (NMDA) receptor ion channel. NMDA receptor plays a key role in central sensitization. The analgesic effects of magnesium are based on blocking the NMDA receptor in the spinal cord (15).
An ideal perioperative analgesia regimen should facilitate not only relief of acute postoperative pain but should also decrease the burden of CPTP. We examined the effect of MgSO4 adjunct to our hospital standard analgetic medication.
Methods
In a prospective, observational pilot study, 50 consecutive patients scheduled for thoracotomy with standard care (control group) should be compared another 50 consecutive thoracotomy patients with adjunct MgSO4 administration (study group). The study was approved by the Ethics Committee of the local medical board (Landesärztekammer Hessen, Germany, MC 144/2013) and was registered at ClinicalTrials.gov (NCT 02008747). Written informed consent was obtained from all patients participating in this trial.
Control group: anesthetic and surgical standard protocol
All patients received a total intravenous general anesthesia (TIVA) using a double lumen intubation. Anesthesia was induced with propofol (1–2 mg/kg) and remifentanil (1 µg/kg). The target was to maintain bispectral index (BIS) value of below 60 during anesthesia induction. If this was not achieved with induction dose of propofol and remifentanil, a bolus of either 0.3 mg kg−1 propofol and/or 0.5 µg kg−1 remifentanil was applicated as appropriate. Anesthesia was maintained with propofol (4–5 mg/kg/h) and remifentanil (0.2–0.5 µg/kg/min). Doses were corrected for ideal body weight (IBW) using the Broca Index. Depth of anesthesia was monitored with a BIS. Target value was between 40 and 60.
Posterolateral thoracotomy was performed in lateral decubitus position. All surgeries were performed by the same surgical team. After completion of the lung resection, each patient had two chest tubes of 28 Ch placed anteriorly and posteriorly, respectively. All patients were extubated at the end of surgery and were transferred to the post-anesthesia care unit (PACU).
Patients had continuous ECG monitoring over 24 h and a 12-lead ECG on postoperative days (POD) 1, 3 and 7.
Analgesic medication was provided according hospital standards which is based on the WHO pain relief ladder (16) using intravenous piritramid patient-controlled-analgesia (first 24 h). Thereafter, oral opioid (oxycodone), metamizol, paracetamol and/or ibuprofen were prescribed depending on the intensity of pain. A pain score of <4 using the NRS was the target.
Study group: MgSO4-administration
Besides the above-mentioned standard care, MgSO4 was added during induction of anesthesia (40 mg/kg over ten minutes), followed by an infusion for 24 hours (10 mg/kg/h). The most significant adverse effect of MgSO4 is hypotension. We recorded every hypotension (MAP <60 mmHg) which had to be treated with catecholamine.
Assessment of pain
Pain was assessed before surgery and on POD 1–8, 30 and 90, respectively. Neuropathic pain was assessed before surgery and POD 3, 7, 30 and 90, respectively.
The pain intensity was assessed using a 10-point NRS, with 0= no pain and 10= worst pain imaginable. For the purpose of the study, values ≥4 were suggested to indicate relevant pain.
The LANSS questionnaire is a validated tool for qualitative pain assessment aimed at assessing neuropathic pain (17). The questionnaire consists of seven items which are summarized to one score with a scaling range between 0 and 24. A LANSS or self-report LANSS (S-LANSS) score ≥12 is considered to be neuropathic pain (18). To assess the chronic neuropathic pain, we used at days 30 and 90 after surgery the S-LANSS, where subjects are instructed to perform self-examinations to determine the presence of neuropathic pain (17). For the purpose of the study, a German translation of the LANSS was used. The incidence and severity of CPTP were assessed by a telephone survey 30 and 90 days after surgery.
Inclusion/exclusion criteria
We included patients aged 18 years or older, who were scheduled for posterolateral thoracotomy. We excluded patients with pregnancy, previous thoracotomy in the medical history, presurgically diagnosed neuropathic pain, hypersensitivity to MgSO4, pre-existing atrial fibrillation, medication with β-blocker and medication with calcium channel blockers, respectively.
Statistics
Descriptive statistics included means, standard deviation (SD), medians and proportions (in %) with 95% confidence intervals as appropriate. Presence of neuropathic pain and the significance between groups was evaluated using χ2 or Fisher exact test, as appropriate. Statistical analysis was performed with SPSS software (SPSS 15.0, Chicago, Illinois, United States). Significance was defined as a P value of <0.05.
Results
One hundred patients were evaluated for study inclusion. All data could be recorded before and within the immediate postoperative period of 8 days. Four patients were lost-to-follow-up. Ninety-six patients completed the telephone questionnaire after 30 POD, and eighty-nine patients after 90 POD. Patient demographic characteristics and types of operation are summarized in Table 1. There were no differences between the two groups with respect to the illustrated variables.
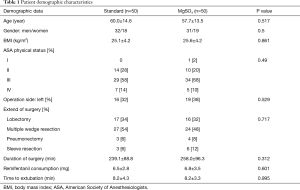
Full table
Patients treated with MgSO4 needed less morphine (Table 2) reaching statistically significance on POD 5 and 30 as well as showing a clear trend on POD 6, 7, 8 and 90.
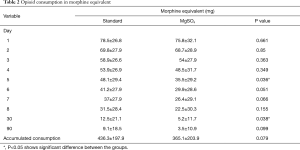
Full table
NRS pain scores at rest were significantly lower in the MgSO4 group at POD 1 to 8, as well as during coughing on POD 4, 7, 8 and 30 (Table 3, P<0.05). Assessed by the NRS pain scores, there was no difference between the two groups on the rest of POD during coughing as well as in the late period on POD 90 at rest and during cough, respectively. There was a clear trend (P=0.079) for less morphine consumption in the MgSO4 group (Table 2).
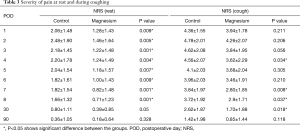
Full table
In the MgSO4 group, less patients had NRS ≥4 (Table 4, Figure 1) at rest reaching significance only on POD 4, 7 and 30, respectively.

Full table

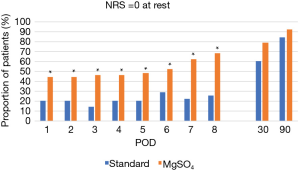
More patients had NRS =0 at rest reaching significance POD 1 to 8, but without significance at POD 30 and 90 in the MgSO4 group compared to the control group (Table 5, Figure 2).

Full table
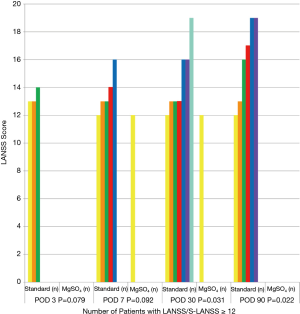
Less neuropathic pain (LANSS score ≥12) was observed in the MgSO4 group (Figure 3) compared to the control group. There was a significant difference on POD 30 and 90, respectively. Nobody reported neuropathic pain (LANSS score ≥12) on POD 90 in the MgSO4 group.
There was no difference in the prevalence of perioperative hypotension (MgSO4 group 18% vs. control group 34%, P=0.61) or conduction block.
Discussion
Acute and chronic pain continues to remain a major problem and a primary concern for patients after thoracotomy despite our increased knowledge in the pathophysiology and pharmacology of nociception (1). Reduction in the incidence and severity of chronic postoperative pain has not occurred although our ability to control acute incisional and inflammatory postoperative pain has increased with the combined use of systemic opioids, regional analgesia technique, and other systemic anti-inflammatory medications (19). The main finding in the present study is that MgSO4 showed analgesic effects. We observed less acute pain at rest on postoperative days 1 to 8 according to the measured numeric pain scores. Assessed by LANSS, chronic neuropathic post-thoracotomy pain was less detected on POD 30 and 90, respectively.
The adjuvant analgesic effect of magnesium after surgery has been described in several reports (20-22). In our study, patients who received MgSO4 during the operation and up to 24 h in total, had less postoperative pain and less opioid consumption compared with patients who did not as shown in previous studies (20). Remifentanil is used in our TIVA protocol. A previous study showed that intraoperative magnesium prevents remifentanil-related hyperalgesia (23). MgSO4 reduces remifentanil induced acute opioid tolerance and hyperalgesia (24). We observed less morphine consumption in the MgSO4 group without reaching statistically significance (P=0.079) but showing clinically relevance in our study. These findings confirm the results of previous studies on the analgesia-potentiating effect of magnesium (20,25). MgSO4 given in a similar setting was reported to have analgesic effect in patients with migraine and postoperative pain (26,27).
Even if MgSO4 was given only for a short-term, neuropathic pain was less frequently in the MgSO4 group. There was a significant difference in the late period. Overall, the rate of neuropathic pain was rather low at all in our study (POD 30 =14.3%, POD 90 =12.2%) compared to previous studies with neuropathic rates reported to range between 40–50% (28-30). The NMDA receptor is involved in the development of sensitization, wind-up, expansion of receptive fields, and neuroplastic changes in the central nervous system (31,32). For the long-term effect, it has been shown that magnesium as physiological blocker of the NMDA calcium channel suppresses neuropathic pain, enhances morphine analgesia, and attenuate morphine tolerance, respectively (33). Moreover, magnesium deficiency produces hyperalgesia that can be reversed by NMDA antagonists (34). This may be another explanation for the effects we could observe in our study. There are also studies in patients with post-therapeutic pain which showed the analgesic effect of MgSO4 on neuropathic pain (35,36).
The concept of pre-emptive analgesia means, that introducing an analgesic regimen before the application of noxious stimuli will prevent sensitization of the nervous system and reduce the incidence and severity of chronic pain (37). Controlling acute postoperative pain is one strategy in avoiding the development of chronic pain. Hypomagnesemia can activate inflammatory neuro-endocrine pathways, and some anti-inflammatory effects of MgSO4 may be due to the treatment of subclinical hypomagnesemia. Additionally, magnesium has α-adrenergic antagonistic effects and inhibits calcium-mediated neuroendocrine secretion (38). Those effects may impact the nociceptive processing and is another explanation of the analgesic effect of magnesium long after primary application.
Several studies have demonstrated less clinical-relevant and severe pain after minimal-invasive surgery (39,40). However, there are still indications for thoracotomy in the era of minimal-invasive thoracoscopic and robotic surgery, respectively. The present study has demonstrated a positive effect on the acute and chronic postoperative pain after open and generally more painful surgery. In the next step, the effects of MgSO4 might be investigated in patients undergoing minimally-invasive surgery in narcotized or awake patients as adjunct to local anesthetics, respectively.
Every study has its limitations. First, it is a prospective observational study, but not a controlled randomized trial. Second, we did not explicitly look at adverse events such as flushing, nausea, headache and dizziness. Third, our patients had no epidural catheter. However, insertion of an epidural catheter has its failure rate which might be linked to inadequate analgesia (41). Inadequate analgesia in the acute postoperative period might be one cause in developing CPTP. No superiority of epidural analgesia could be found in a large retrospective trial with 1,555 thoracotomies (42) comparing epidural analgesia with oral opioid protocol. Patients with epidural analgesia were dismissed with higher opioid doses than patients with the oral opioid protocol. No differences in recovery of bowel function were found. This underlines the need of an effective analgesic concept in the perioperative setting. Finally, it was not a double-blinded study which is important for the objective assessment of pain.
In conclusion, our findings demonstrate that MgSO4 yields substantial benefit in reduction and preventing acute postoperative pain at rest as well as chronic neuropathic post-thoracotomy pain. MgSO4 reduces the postoperative opioid consumption. Further randomized-controlled clinical trials are needed to confirm these findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was conducted in accordance with the amended Declaration of Helsinki. Local Ethics Committee approved the protocol (MC 144/2013) and the study was registered at ClinicalTrials.gov (NCT 02008747). Written informed consent was obtained from all patients participating in this trial.
References
- Maxwell C, Nicoara A. New developments in the treatment of acute pain after thoracic surgery. Curr Opin Anaesthesiol 2014;27:6-11. [Crossref] [PubMed]
- Maguire MF, Ravencroft A, Beggs D, et al. A questionnaire study investigating the prevalence of the neuropathic component after thoracic surgery. Eur J Cardiothorac Surg 2006;29:800-5. [Crossref] [PubMed]
- Derrode N, Lebrun F, Levron JC, et al. Influence of preoperative opioid on postoperative pain after major abdominal surgery: sufentanil TCI versus remifentanil TCI. A randomized controlled study. Br J Anaesth 2003;91:842-49. [Crossref] [PubMed]
- Wu L, Huang X, Sun L. The efficacy of N-methyl-D-aspartate receptor anatgonists on improving the postoperative pain intensity and satisfaction after remifentanil-based anesthesia in adults: a meta-analysis. J Clin Anesth 2015;27:311-24. [Crossref] [PubMed]
- Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2000;18:711-6. [Crossref] [PubMed]
- Sentürk M, Ozcan PE, Talu GK, et al. The effects of three different analgesia techniques on longterm postthoracotomy pain. Anesth Analg 2002;94:11-5. [Crossref] [PubMed]
- Wallace AM, Wallace MS. Post-mastectomy and post thoracotomy pain. Anesthesiol Clin North Am 1997;15:353-70. [Crossref]
- Dajczman E, Gordon A, Kreisman H, et al. Long-term postthoracotomy pain. Chest 1991;99:270-4. [Crossref] [PubMed]
- Benedetti F, Vighitti S, Amanzino M, et al. Dose-response relationship of opioids in nociceptive and neuropathic postoperative pain. Pain 1998;74:205-11. [Crossref] [PubMed]
- Maruta T, Kodama Y, Tanaka I, et al. Comparison of the effect of continuous intravenous infusion and two bolus injections of remifentanil on haemodynamic responses during anaesthesia induction: a prospective randomised single-centre study. BMC Anesthesiol 2016;16:110. [Crossref] [PubMed]
- Alanoğlu Z, Tolu S, Yalcin S, et al. Different remifentanil doses in rapid sequence anesthesia induction: BIS monitoring and intubation conditions. Adv Clin Exp Med 2013;22:47-55. [PubMed]
- Witkowska M, Karwacki Z, Rzaska M, et al. Comparison of target controlled infusion and total intravenous anaesthesia with propofol and remifentanil for lumbar microdiscectomy. Anaesthesiol Intensive Ther 2012;44:138-44. [PubMed]
- Sihoe AD, Lee TW, Wan IY, et al. The use of gabapentin for post-operative and post-traumatic pain in thoracic surgery patients. Eur J Cardiothorac Surg 2006;29:795-9. [Crossref] [PubMed]
- Vanstone RJ, Rockett M. Use of atypical analgesics by intravenous infusion (IV) for acute pain: evidence base for lidocaine, ketamine and magnesium. Anesthesia and Intensive Care Med 2016;17:460-3. [Crossref]
- Srebro D, Vuckovic S, Milovanovic A, et al. Magnesium in pain research: state of the art. Curr Med Chem 2016. [Epub ahead of print]. [PubMed]
- Available online: https://www.who.int/cancer/palliative/painladder/en
- Bennett MI, Smith BH, Torrance N, et al. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 2005;6:149-58. [Crossref] [PubMed]
- Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001;92:147-57. [Crossref] [PubMed]
- Buchheit T, Pyati S. Prevention of chronic pain after surgical nerve injury: amputation and thoracotomy. Surg Clin North Am 2012;92:393-407. [Crossref] [PubMed]
- Albrecht E, Kirkham KR, Liu SS, et al. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. Anaesthesia 2013;68:79-90. [Crossref] [PubMed]
- De Oliveira GS Jr, Castro-Alves LJ, Khan JH, et al. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2013;119:178-90. [Crossref] [PubMed]
- Guo BL, Lin Y, Hu W, et al. Effects of systemic magnesium on post-operative analgesia: is the current evidence strong enough? Pain Physician 2015;18:405-18. [PubMed]
- Song JW, Lee YW, Yoon KB, et al. Magnesium sulfate prevents remifentanil-induced postoperative hyperalgesia in patients undergoing thyroidectomy. Anesth Analg 2011;113:390-7. [Crossref] [PubMed]
- Schug SA, Palmer GM, Scott DA, et al. APM: SE Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute pain management: scientific evidence. 4th edition. Melbourne: ANZCA&FPM, 2015.
- Hwang JY, Na HS, Jeon YT, et al. I.V. infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. Br J Anaesth 2010;104:89-93. [Crossref] [PubMed]
- Xiao WH, Bennett GJ. Magnesium suppresses neuropathic pain responses in rats via spinal site of action. Brain Res 1994;666:168-72. [Crossref] [PubMed]
- Mauskop A, Altura BT, Cracco RQ, et al. Intravenous magnesium sulfate relieves migraine attacks in patients with low serum ionized magnesium levels: a pilot study. Clin Sci 1995;89:633-6. [Crossref] [PubMed]
- Kinney MA, Hooten WM, Cassivi SD, et al. Chronic postthoracotomy pain and health-related quality of life. Ann Thorac Surg 2012;93:1242-7. [Crossref] [PubMed]
- Pluijms WA, Steegers MA, Verhagen AF, et al. Chronic post-thoracotomy pain: a retrospective study. Acta Anaesthesiol Scand 2006;50:804-8. [Crossref] [PubMed]
- Guastella V, Mick G, Soriano C, et al. A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain 2011;152:74-81. [Crossref] [PubMed]
- Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis 1998;5:209-27. [Crossref] [PubMed]
- Kvarnström A, Karlsten R, Quiding H, et al. The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol Scand 2003;47:868-77. [Crossref] [PubMed]
- McCarthy RJ, Kroin JS, Tuman KJ. Antinociceptive potentiation and attenuation of tolerance by intrathecal co-infusion of magnesium sulfate and morphine in rats. Anesth Analg 1998;86:830-6. [Crossref] [PubMed]
- Weissberg N, Schwart G, Shemesh O. Serum and intracellular electrolytes in patients with and without pain. Magnes Res 1991;4:49-52. [PubMed]
- Brill S, Sedgwick PM, Hamann W, et al. Efficacy of intravenous magnesium in neuropathic pain. Br J Anaesth 2002;89:711-4. [Crossref] [PubMed]
- Kim YH, Lee PB, Kyu T. Is magnesium sulfate effective for pain in chronic postherpetic neuralgia patients comparing with ketamine infusion therapy? J Clin Anesth 2015;27:296-300. [Crossref] [PubMed]
- Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology 2002;96:725-41. [Crossref] [PubMed]
- James MFM. Magnesium an emerging drug in anesthesia. Br J Anaesth 2009;103:465-7. [Crossref] [PubMed]
- Mongardon N, Pinton-Gonnet C, Szekely B, et al. Assessment of chronic pain after thoracotomy: a 1-year prevalence study. Clin J Pain 2011;27:677-81. [Crossref] [PubMed]
- Wildgaard K, Ravn J, Nikolajsen L, et al. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand 2011;55:60-8. [Crossref] [PubMed]
- Pratt WB, Steinbrook RA, Maithel SK, et al. Epidural analgesia for pancreatoduodenectomy: a critical appraisal. J Gastrointest Surg 2008;12:1207-20. [Crossref] [PubMed]
- Kampe S, Weinreich G, Darr C, et al. The impact of epidural analgesia compared to systemic opioid-based analgesia compared to systemic opioid-based analgesia with regard to length of hospital stay and recovery of bowel function: retrospective evaluation of 1555 patients undergoing thoracotomy. J Cardiothorac Surg 2014;9:175. [Crossref] [PubMed]


