
Age is an independent predictor in pathological diagnosis of sarcoidosis: a retrospective analysis of diagnosis by endobronchial ultrasound-guided transbronchial needle aspiration
Introduction
Sarcoidosis is a multisystem disorder of unknown etiology. The diagnosis of sarcoidosis is established on the basis of compatible clinical and radiologic findings, supported by pathological findings which is typically noncaseating epithelioid-cell granulomas in one or more organs (1,2). Previous epidemiological studies have reported that thoracic lesions, including hilar and mediastinal lymphadenopathy and/or pulmonary parenchymal lesions, are most frequently observed in patients with sarcoidosis (3,4). Thus, pathological evaluation of thoracic lesions is important for a definitive diagnosis.
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a useful modality for the diagnosis of thoracic lymphadenopathy, including primary lung cancer, malignant lymphoma, and sarcoidosis (5). Before the development of EBUS-TBNA, transbronchial lung biopsy (TBLB) by conventional bronchoscopy was the common method for pathological diagnosis of sarcoidosis. However, a recent meta-analysis showed that the diagnostic yield of sarcoidosis by EBUS-TBNA was significantly higher than by conventional TBLB (6). Another review article also showed high sensitivity and safety of EBUS-TBNA for the pathological diagnosis of sarcoidosis. For example, the diagnostic yield of EBUS-TBNA for a patient with sarcoidosis with hilar or/and mediastinal lymphadenopathy ranged from 54% to 93%, and the diagnostic accuracy was 79% (95% CI, 71–86%) (7). Thus, EBUS-TBNA is the major modality for the diagnosis of thoracic sarcoidosis.
Few studies have evaluated the predictors for a pathological diagnosis of sarcoidosis by EBUS-TBNA. One retrospective study showed no association between the size of the lymph node and number of passes (8). Another prospective study demonstrated that the short-axis diameter of lymph node, stage of disease, and number of passes were independent predictive factors associated with positive pathological findings of sarcoidosis (9). Results from these previous reports suggest that investigating the predictors for pathological diagnosis of sarcoidosis by EBUS-TBNA remain insufficient. Therefore, the objective of this study was to further assess a novel predictor for the pathological diagnosis of sarcoidosis by EBUS-TBNA.
Methods
We conducted a single-center retrospective study in the Department of Respiratory Medicine, Juntendo University Hospital (Tokyo, Japan). The study protocol was approved by the independent ethics committee of the institutional review board in Juntendo University Hospital (No. 17-125).
Patients were selected who were pathologically and/or clinically diagnosed with sarcoidosis from 631 patients who had undergone EBUS-TBNA between February 2010 and December 2017. Sarcoidosis was diagnosed based on the criteria of “Diagnostic Standard and Guideline for Sarcoidosis 2015” established by the Japan Society of Sarcoidosis and other Granulomatous Disorders. Patient with a “pathological” or “clinical” diagnosis of sarcoidosis based on these criteria were enrolled in this study. Systemic evaluations for sarcoidosis to detect other organ’s lesions involving ocular, heart and brain were performed in pathological positive group and clinically highly suspected group.
EBUS-TBNA was performed using a convex endobronchial ultrasound bronchoscope probe (BF-UC260FW; Olympus, Tokyo, Japan) under the sedation by pethidine (17.5 mg/body) and midazolam (2–4 mg/body) in all enrolled patients. A dedicated 22-gauge needle (NA-201SX-4022; Olympus, Tokyo, Japan) was used for the punctures. EBUS-TBNA was performed without rapid on-site evaluation of samples. All procedures were performed as previously described (10).
After evaluation of characteristics, patients and punctured lymph nodes were divided into groups of pathological positive and negative by EBUS-TBNA. We defined positive pathological findings as epithelioid granuloma without caseous necrosis. Univariate analyses of patients were performed on variables of age (5 years was regarded as 1 unit), gender, stage, total number of punctures per procedure, and the number of punctured lymph nodes. In addition, univariate analyses of punctured lymph nodes were performed on stage, short and long-axis diameter of the target lymph node, and total number of punctures per lymph node. We chose these parameters by referring to previous studies. We also included some parameters of basic characteristics. The short and long-axis diameters of the lymph node were measured using computerized tomography.
We used t-test to compare continuous variables and chi-square tests for categorical variables. Multivariate logistic regression analysis was subsequently performed for parameters that reached P<0.10 in the univariate analyses in our study and factors that have been associated with a pathological positive diagnosis of sarcoidosis by EBUS-TBNA in previous studies. These analyses were performed using Statistical Package for Social Science (SPSS) Version 19 software (Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
We selected 93 patients finally diagnosed with sarcoidosis, and excluded four patients who did not have satisfactory data about evaluated parameters from 631 patients who had an EBUS-TBNA between February 2010 and December 2017 (Figure 1).
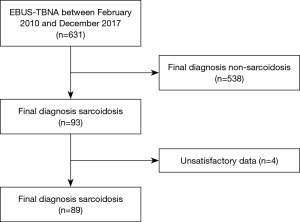
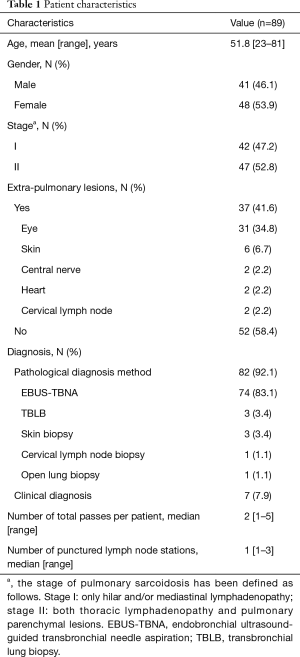
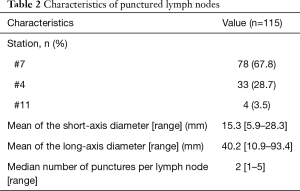
Characteristics of the 89 patients are shown in Table 1. Among the 89 patients (42 with stage I and 47 with stage II), 82 patients (92.1%) were pathologically diagnosed, and 74 of those patients (83.1% of the total) were diagnosed by EBUS-TBNA. Transbronchial lung, skin, cervical lymph node, and open lung biopsies were used for diagnosis in eight patients. A total of 115 mediastinal and hilar lymph nodes were punctured in these 89 patients. Characteristics of these lymph nodes are shown in Table 2. Stations of the punctured lymph nodes were #7 (67.8%), #4 (28.7%), and #11 (3.5%).
Full table
Full table
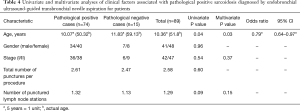
The mean short-axis and long-axis diameter was 15.5 and 14.7 mm, and 40.5 and 39.3 mm in the pathological positive and negative groups, respectively. There were no statistically significant differences in the size of the lymph node between groups. Statistical analysis also showed no significant differences in the number of punctures performed per each lymph node and stage of disease between the groups (Table 3).

Full table
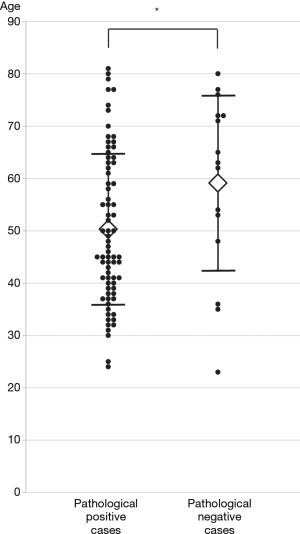
While gender, stage, total number of passes during the procedure, and number of punctured lymph node stations were not significantly associated with pathological positive findings by EBUS-TBNA, age was significantly related to the positive findings of sarcoidosis by univariate analysis. Furthermore, multivariate logistic regression revealed that age was significantly associated with pathological positive findings by EBUS-TBNA [5 years was regarded as 1 unit, odds ratio (OR), 0.79; 95% CI, 0.64–0.97; P=0.03] (Table 4). This result showed that the diagnostic yield of sarcoidosis by EBUS-TBNA was significantly higher in younger than older patients. Actual age of enrolled patients both in pathological positive and negative groups were shown in Figure 2.
Full table
Discussion
In this study, we have assessed the predictive factors of positive pathological findings in sarcoidosis by EBUS-TBNA. First, we have shown that age was an independent predictor in the pathological diagnosis of sarcoidosis by EBUS-TBNA. Furthermore, age was significantly lower in pathological positive group than in negative group. Several parameters, which are associated with pathological positive findings by EBUS-TBNA in previous studies, were not predictors in this study.
Previously, a retrospective study has reported that needle gauge, size of lymph node, total number of needle passes, and number of lymph node stations sampled is not associated with the positive pathological findings of sarcoidosis by EBUS-TBNA; however, the skill of the operator was related to pathological diagnosis (8). In contrast, another prospective study has suggested that short axis, >1 pass per lymph node, and stage I diagnosis are independent predictive factors associated with diagnostic yield by EBUS-TBNA (9). Additionally, Oki et al. have recently reported that ≥4 passes per patient for either single or multiple lesions was recommended for successful pathological diagnosis in sarcoidosis by EBUS-TBNA (11). Similar to our study, these reports evaluated the predictive factors in pathological diagnosis by EBUS-TBNA; however, the results are not completely consistent and predictive factors remain uncertain. Moreover, these studies did not evaluate age as a predictor for successful pathological diagnosis of sarcoidosis by EBUS-TBNA.
This study showed that the age of pathological positive group by EBUS-TBNA was significantly lower than the negative group. This result may be explained using the biological and epidemiological backgrounds of sarcoidosis. The pathogenesis of sarcoidosis is a granulomatous immune reaction to environmental antigens, such as Propionibacterium acnes or Mycobacterium species, mainly caused by type 1 helper T cells (Th-1) (12). Antigen-presenting cells, including macrophages and dendritic cells, cause an exaggeration of Th-1 cell proliferation, which results in intra- and/or extra-thoracic granulomatous formations (13). Moreover, recent investigations suggested the modulatory function of regulatory T cells (Tregs) in the immune reaction of sarcoidosis. A previous study indicated that the number of Tregs in an elderly group was significantly increased when compared with a younger group in an animal model, and there was a direct correlation between the expansion of Tregs and immune deficiency in aged animals (14). A recent epidemiological study in Japan showed that the incidence of thoracic lymphadenopathy was higher in a patient group <45 years of age, suggesting a progressive decline in immune response with age (15). This may lead to lower diagnostic yield of sarcoidosis by EBUS-TBNA in older patients. Therefore, our results strongly recommend the use of EBUS-TBNA for younger patients in the diagnosis of sarcoidosis.
Conversely, a recent epidemiological study showed that age at diagnosis has gradually increased over the past four decades in Japan and western countries (3,4). Reduced exposure to microbial agents in early life, which could elevate the risk of developing sarcoidosis, may reflect the increasing age of disease onset in recent decades. Interestingly, the age at diagnosis of sarcoidosis in this study was relatively high compared with previous reports, even in the pathological positive group; therefore, the onset and age at diagnosis of sarcoidosis may increase in the near future.
Several limitations of this study should be mentioned. First, this study was a retrospective analysis with a small number of patients in single institution, which may account for the discrepancy in results between this and previous studies in the analysis of predictive factors. Second, we have speculated about differences in immune response that are dependent on age accounting for our results; however, we have not assessed immune reactivity of patients in this study, so this cannot be confirmed. Third, we have not verified all previously evaluated predictors. For example, a previous prospective study indicated that ≥4 passes can lead to a pathological positive (11), the mean number of passes in this study was less than that of the previous report. Moreover, we could not assess the skill of the operator on effective pathological diagnosis. Fourth, this study had assessed only Japanese patients. Frequency of sarcoidosis probably depends on each ethnicity. Further prospective investigation including variety of ethnicity is needed in the future.
In summary, we have investigated predictive factors for the pathological diagnosis of sarcoidosis by EBUS-TBNA. The diagnostic yield of sarcoidosis by EBUS-TBNA was higher in younger than older patient; therefore, age may be an independent predictor for the pathological diagnosis of sarcoidosis by EBUS-TBNA. We may have to select the modality in the diagnosis of sarcoidosis depending on patient’s age.
Acknowledgments
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the independent ethics committee of the institutional review board in Juntendo University Hospital (No. 17-125).
References
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007;357:2153-65. [Crossref] [PubMed]
- Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med 2011;183:573-81. [Crossref] [PubMed]
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164:1885-9. [Crossref] [PubMed]
- Sawahata M, Sugiyama Y, Nakamura Y, et al. Age-related and historical changes in the clinical characteristics of sarcoidosis in Japan. Respir Med 2015;109:272-8. [Crossref] [PubMed]
- Medford AR, Bennett JA, Free CM, et al. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): applications in chest disease. Respirology 2010;15:71-9. [Crossref] [PubMed]
- Hu LX, Chen RX, Huang H, et al. Endobronchial Ultrasound-guided Transbronchial Needle Aspiration versus Standard Bronchoscopic Modalities for Diagnosis of Sarcoidosis: A Meta-analysis. Chin Med J (Engl) 2016;129:1607-15. [Crossref] [PubMed]
- Agarwal R, Srinivasan A, Aggarwal AN, et al. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med 2012;106:883-92. [Crossref] [PubMed]
- Navasakulpong A, Auger M, Gonzalez AV. Yield of EBUS-TBNA for the diagnosis of sarcoidosis: impact of operator and cytopathologist experience. BMJ Open Respir Res 2016;3:e000144. [Crossref] [PubMed]
- Sun J, Yang H, Teng J, et al. Determining factors in diagnosing pulmonary sarcoidosis by endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Surg 2015;99:441-5. [Crossref] [PubMed]
- Nakajima T, Yasufuku K. How I do it--optimal methodology for multidirectional analysis of endobronchial ultrasound-guided transbronchial needle aspiration samples. J Thorac Oncol 2011;6:203-6. [Crossref] [PubMed]
- Oki M, Saka H, Ando M, et al. How Many Passes Are Needed for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Sarcoidosis? A Prospective Multicenter Study. Respiration 2018;95:251-7. [Crossref] [PubMed]
- Chen ES, Moller DR. Etiologies of Sarcoidosis. Clin Rev Allergy Immunol 2015;49:6-18. [Crossref] [PubMed]
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet 2014;383:1155-67. [Crossref] [PubMed]
- Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol 2006;177:8348-55. [Crossref] [PubMed]
- Sawahata M, Sugiyama Y, Nakamura Y, et al. Age-related differences in chest radiographic staging of sarcoidosis in Japan. Eur Respir J 2014;43:1810-2. [Crossref] [PubMed]


