
Treatment of symptomatic intercostal heterotopic ossification after surgical stabilization of rib fractures: report of two cases and review of the literature
Introduction
Heterotopic ossification (HO) is the abnormal growth of bone in soft tissue outside of the usual skeletal anatomical locations. This condition should not be confused with metastatic or dystrophic calcification, both of which result from calcium deposition in soft tissues rather than from the transformation of primitive mesenchymal cells into osteogenic cells, resulting in the growth of true mature lamellar bone, as is seen in HO (1-3). HO can cause swelling, limitations in range of motion secondary to ankylosis, and pain secondary to peripheral nerve entrapment, all of which contribute to significant long term disability. Although the pathophysiology of HO is complicated and poorly understood, identifiable precipitants include traumatic musculoskeletal injuries (e.g., fractures, dislocations, orthopedic surgeries, etc.), central nervous system (CNS) injuries, and, rarely, genetic diseases (4). Orthopedic surgeries, including joint arthroplasty and surgical fixation of upper and lower extremity fractures, are some of the most widely reported operations linked to HO; however, to our knowledge, HO following fixation of traumatic rib fractures has not previously been reported in the literature.
Prophylaxis against and treatment of HO may consist of a combination of range of motion exercises, bisphosphonates (e.g., etidronate), nonsteroidal anti-inflammatory drugs (e.g., indomethacin), and local radiation therapy, although there is no true consensus among existing orthopedic literature as to what treatment should be used and when therapy should begin. Treatment may also involve surgical resection of the heterotopic bone segments, although this is usually reserved for patients with severely limited range of motion, as HO is likely to recur and progress if the resected bony lesion had not yet fully matured (1).
In this paper, we present the cases of two patients who presented at our institution with bony bridging between formerly fractured and internally fixated ribs and the methods we used to treat them.
Case presentation
Case 1
GK is a 52-year-old male contractor who presented to our Emergency Department after sustaining a fall from a 20-foot ladder. Workup revealed multiple blunt trauma injuries including right-sided flail chest and right-sided pneumothorax. He then underwent open reduction and internal fixation utilizing anterior osteosynthesis plates and bicortical screws (Matrix RIBTM Fixation System, DePuy Synthes) of ribs 5 and 6 anteriorly and ribs 5, 6, and 8 posteriorly. He was discharged on post-op day 4 with minimal pain and full mobility.
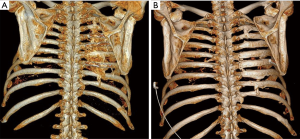
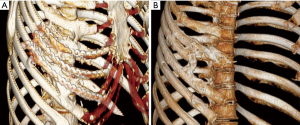
At 3-month follow-up, the patient presented to trauma clinic with severe neuropathic pain with hypesthesia and decreased rotation range of motion of the torso. A CT scan done at that time showed new bone growth between the posterior aspects of ribs 4–8 (Figure 1). At 6 months the patient underwent surgical removal of hardware along with excision of the boney masses followed by a single pulse of external beam radiation (EBR) at 7 Gy and two months of oral indomethacin. Intraoperatively, he was noted to have a carapace-like ossification which was resected while keeping the hardware in place to act as a guide as the normal anatomy of the ribs had been completely obliterated (Figure 2). Six months after this surgery the patient reported greatly reduced pain and full range of motion. A CT scan at this time showed no return of hyperostosis (Figure 1). He then initiated intercostal nerve blockade therapy with concurrent physical therapy to address his remaining chronic pain.

Case 2
KB was a 50-year-old female transferred from an outside hospital after a boating accident in which she fell overboard and was struck by another fast-moving boat. This patient presented with multiple blunt traumatic injuries including a right-sided anterolateral unstable chest wall involving ribs 2–9. She was admitted for management of her condition and on day 11 of hospitalization she underwent open reduction and internal fixation of her right-sided rib fractures utilizing anterior osteosynthesis plates and bicortical screws (Matrix RIBTM Fixation System, DePuy Synthes). Following her discharge, patient was lost to follow-up for two years.
When the patient presented to trauma clinic two years later, she complained of debilitating chest wall pain and persistent restriction of mobility of the thorax. A CT scan done at that time revealed bony bridging between ribs 2–6 on the right side (Figure 3). She then underwent surgical hardware removal and excision of the majority of bony bridging followed by a single pulse of prophylactic EBR (7 Gy) and two months of oral indomethacin. Six months following her surgery the patient reported decreased pain and increased mobility of her thorax. CT scan confirmed no recurrence of HO. Of note, one bony bridge between ribs 4 and 5 anteriorly was left behind purposefully. Imaging suggested resorption of part of the 4th rib anterior to the site of HO, and intraoperative assessment suggested that resection of that bridge might result in chest wall instability in that area, therefore the decision was made to leave that one area of bony bridging intact. Despite this incomplete resection, the patient did have complete resolution of symptoms at last clinic visit.
Histological analysis of the excised bone in both cases showed cellular bone marrow with trilineage hematopoiesis and no histological features of infection or malignancy, confirming the presence of true HO rather than a condition involving calcification processes.
Overview of HO
HO is the presence of lamellar bone in soft tissue structures where bone typically does not exist (1). HO was first termed in 1883 by Reidel (2); however, documented references to the condition date back to the 10th century, when Al-Zahrawi described complications in bone healing related to tissue calcification that impeded normal limb functioning (3). In the modern medical literature, terms denoting other abnormal bone-forming conditions, including ectopic ossification, myositis ossificans, neurogenic ossifying fibromyopathy, and paraosteoarthropathy or periarticular ossification, have also been used to denote HO (1,2). As the etiologies of these other conditions have been further investigated and formally defined, HO has become the term used across current medical literature to denote this unique and still elusive condition (1).
HO has a high correlation with trauma, particularly with trauma to the musculoskeletal and CNSs, and it is among the post-injury and post-surgical complications that can greatly impede patient recovery and rehabilitation (3). HO can present in a variety of ways, but in the acute setting it is characterized by fever, swelling, erythema, and decreased or limited joint motion, around the affected body region (1,2,4). As these presentations are also symptomatic of other conditions, in particular bone or soft tissue infection, diagnostic certainty, successful prevention, and effective treatment of HO is an ongoing concern of clinicians who treat bony injuries (1,2,4).
Classification
HO is classified into three etiologies: traumatic, neurogenic, and genetic (1,4). Traumatic HO is the most prevalent sub-classification and can result from fractures, dislocations, and operative procedures that injure the musculoskeletal system (4). Following total hip arthroplasty (THA), for instance, HO incidence rates have been reported between 16–53% (1). Post-operative HO occurrence is also attributed to open reduction internal fixation (ORIF), total knee arthroplasty (TKA), and elbow replacements (3). Neurogenic HO (also termed neurological HO) occurs after neurological insult such as spinal cord injury (SCI) and traumatic brain injury (TBI) (2,5). Evidence suggests that neurogenic HO affects 20–29% of patients with SCI and 5–20% of patients with TBI (5). Although SCI and TBI are both experienced by civilians, neurogenic HO is particularly common following combat-related trauma that affects the CNS. A study that examined the prevalence of HO following combat-related trauma reported 86% of patients who experienced blast-related TBI developed neurogenic HO (68% developed traumatic HO) (6).
Genetic HO is extremely rare and associated with genetic disorders such as fibrodysplasia ossificans progressiva, progressive osseous heteroplasia, or Albright hereditary osteodystrophy and is characterized by congenital skeletal abnormalities that progress to debilitating morbidity by early adulthood (2).
Clinical presentation
Symptoms of HO may present as early as 3 weeks or as late as 3 months after a trauma, neurological injury, or precipitating event (1,2,5). Some studies suggest that men are more prone to HO, since they are more likely to experience SCI and TBI, while other studies have countered that there are no gender or racial biases in prevalence (5).
Principal complications of HO are ankylosis and decreased joint mobility (10–20% of HO cases) (2,3). HO can develop at any site, but the most common site is the hip, followed by the elbow, knee, or shoulder, and even more rarely, the wrist, ankle, hand, and foot (2,7,8). General symptoms of HO are pain, warmth, swelling, erythema, and tissue breakdown around joints, bone fusion, and nerve entrapment (1-3). Because of these symptoms, early-course HO may mimic or be confused with other conditions such as cellulitis, thrombophlebitis, osteomyelitis, or the bone-forming tumors osteosarcoma and osteochondroma.
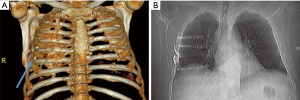
The precise incidence and clinical impact of HO in the thoracic skeleton following injury is unknown. In large part because radiographic detection is exceedingly difficult utilizing simple plain chest X-rays. The symptoms of symptomatic intercostal HO are vague enough (pain, swelling, limited chest range of motion) that practitioners may often assume they represent lingering pain from rib fractures themselves and rarely proceed beyond a plain chest X-ray in order to rule out the more common conditions such as pleural effusion, empyema, pneumothorax, or pneumonia. The irregular bony bridges are thin enough that they typically do not appear visible on a standard X-ray (Figure 4). And because few patients receive CT scans of their chest beyond the first three weeks after injury, when HO becomes evident, it is rarely noted or detected if in symptomatic individuals. For all of these reasons, a comprehensive observational cohort study would be necessary in order to properly assess the magnitude of this disease entity.
Pathophysiology
HO develops outside the joint capsules; bone forms in the connective tissue and between the muscle planes, but the bone does not progress into the muscle itself (8). The cellular and molecular mechanisms that guide the formation of HO, as opposed to normal skeletal development or bone healing, are not entirely understood (7). Still, it is thought the HO has a unique inciting pathophysiology, one that is fundamentally different from that of metastatic or dystrophic ossification. The leading assumption, put forth in the 1970s by Chalmers et al., is that certain trigger mechanisms within the septa of the connective tissue initiates the transformation of primitive mesenchymal cells into osteogenic cells (7,9).
It has been postulated that a protein may be released from healthy bone in response to disease or trauma, and it may act as a causative agent in cell transformation (10). The presence of a centrally mediated factor, prostaglandin E2, or elevated levels of osteoblast-stimulating factors in sera may contribute to cell transformation (11). More broadly, other inducing factors of HO may include hypercalcemia, tissue hypoxia, changes in sympathetic nerve activity, prolonged immobilizing, remobilization, and disequilibrium of parathyroid hormone and calcitonin (12).
Lastly, it should be noted that no clear association has been made between the presence of implanted surgical hardware itself and the development of HO. Of note, patient GK had extensive, carapace-like symptomatic intercostal HO over the entire posterior area affected by rib fractures. Not only was there the presence of bony bridging between ribs that had not been plated, there was also complete absence of bony bridging between the anterior ribs that did have osteosynthesis plates in place (Figure 4).
Screening and detection
The histologic properties of HO are an ossified periphery and a non-ossified center (2). Imaging technology can aid in the detection of HO development and differentiate it from other conditions such as soft tissue infections. Three-phase bone scintigraphy is the most sensitive technology for detection, typically showing increased osteoblastic metabolic activity approximately 2–4 weeks after injury (13). It is during this acute formation phase that resection should not be attempted, as recurrence is significantly higher. Serial bone scans have been used to monitor the activity of HO, in order to plan surgical resection and, when indicated, potentially predict the likelihood of recurrence (14). Conventional radiography, magnetic resonance imaging (MRI), and computer tomography (CT) scans can be used for early detection of HO, but with far less specificity than three-phase bone scintigraphy (1,2). Conventional radiography can detect HO lesions 4-6 weeks after a positive three-phase bone scan (13).
Prophylaxis and treatment
Prevention strategies for HO are limited, given the unique practicality and safety challenges associated with treating trauma patients. Pharmaceutical therapy has been used for the prophylaxis of HO; however, this approach is debatable and contingent on several factors, particularly if polytrauma is involved (14). Non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin and ibuprofen have been used as a prophylactic approach for patients undergoing THA (15). NSAIDs have the two-fold effect of inhibiting the transition of mesenchymal cells into osteogenic cells and suppressing the mechanism that indices post-traumatic bone remodeling (2,15). Radiation therapy has also been successful as a prophylaxis in THA patients, also for its ability to disrupt cell differentiation; however, its efficacy with SCI and TBI patients has not been established (13,14). Radiation is typically administered preoperatively within 24 hours, at a prescribed dosage of 7–8 Gy single fraction, although there is great variation in acceptable fractionations and dosage regimens (1).
Once a diagnosis is confirmed, passive range-of-motion exercises are useful as an early treatment for HO in order to maintain joint mobility (2). If symptoms progress to severe functional impairment or extreme pain, surgical excision (or re-excision) of bone is often a necessary treatment. The optimal timing of surgery is controversial and often associated with secondary complications and a high risk of HO recurrence particularly when HO is in the acute stage of development. Best practices currently dictate to prioritize functional outcomes over the maturity of the bone. Other authors suggest a simple algorithm for the treatment of HO based on the etiology, and in the case of HO resection after direct traumatic injury their recommendation is 6 months (2). Naturally, delay in surgery must be weighed against the interval development of complications of reduced mobility and prolongation of pain.
Postoperative treatment for HO can also utilize both pharmaceutical and radiation therapies. NSAIDs administered postoperatively can minimize the risk of recurrence while also treat the postoperative or post-injury pain (14,15). Bisphosphonates, particularly sodium etidronate, can also inhibit the process of bone calcification. Treatment is effective when started as early as possible and continued for at least 6 months (16). Similar to its prophylactic role, radiation therapy has had well-documented efficacy as postoperative treatment for THA patients who developed HO and underwent resection, but this is understudied as treatment in the broader patient population that develops HO, particularly patients with SCI and TBI (2,3,14). Radiation is typically administered postoperatively within 72 hours of resection at a recommended dosage of 7–8 Gy single fraction (15).
Compared to NSAIDs, radiation therapy has demonstrated a higher efficacy in minimizing recurrence of HO in patients who have undergone THA (14). Additionally, radiation therapy may be a more effective treatment option for patients who have undergone previous surgeries or have contradictions to NSAIDs. However, radiation therapy can result in additional pain and fatigue, wound healing delays and complications, or, in rare instances, elevate risks of secondary cancer.
Conclusions
Although treatment of symptomatic intercostal HO after surgical stabilization of rib fractures has not previously been reported, the vague nature of the symptoms in this condition likely means it has been overlooked in many instances and its true incidence is unknown. As more surgical interventions are employed to treat severe injuries of the chest wall, including fixation with osteosynthesis plates, more CT imaging will be employed to rule out hardware causes of post-operative symptomatology. It is likely, therefore, that HO will be encountered more often and surgeons will look to methods of treatment in select patients where intercostal bridging results in disability. Our small case series suggests that utilizing the treatment algorithm employed by orthopedists of combined surgical resection and post-operative prophylactic NSAIDs and EBR is a viable option until HO itself in this patient population is more carefully examined and treatment as well as prophylaxis is more specifically developed.
Acknowledgements
Special thanks to Dr. Robin McGoey, MD for assistance with interpretation of histological analysis.
Footnote
Conflicts of Interest: Dr. P Greiffenstein has served as a paid consultant on expert panels on product development for surgical devices for KLS Martin, DuPuy Synthes, and Zimmer Biomet. He has taught rib fracture fixation courses for KLS Martin and DuPuy Synthes and teaches rib fracture fixation skills labs for Zimmer Biomet on a regular basis (2–4 times per year). The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med 2005;37:129-36. [Crossref] [PubMed]
- Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med 2002;43:346-53. [PubMed]
- Juarez JK, Wenke JC, Rivera JC. Treatments and Preventative Measures for Trauma-Induced Heterotopic Ossification: A Review. Clin Transl Sci 2018;11:365-70. [Crossref] [PubMed]
- Garland DE. A clinical perspective on common forms of acquired heterotopic ossification. Clin Orthop 1991.13-29. [PubMed]
- Brady RD, Shultz SR, McDonald SJ, et al. Neurological heterotopic ossification: Current understanding and future directions. Bone 2018;109:35-42. [Crossref] [PubMed]
- Forsberg JA, Pepek JM, Wagner S, et al. Heterotopic ossification in high-energy wartime extremity injuries: Prevalence and risk factors. J Bone Joint Surg Am 2009;91:1084-91. [Crossref] [PubMed]
- Kraft CT, Agarwal S, Ranganathan K, et al. Trauma-induced heterotopic bone formation and the role of the immune system: A review. J Trauma Acute Care Surg 2016;80:156-65. [Crossref] [PubMed]
- Jensen LL, Halar E, Little J, et al. Neurogenic heterotopic ossification. Am J Phys Med 1987;66:351-63. [PubMed]
- Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br 1975;57:36-45. [Crossref] [PubMed]
- Urist MR, Nakagawa M, Nakata N, et al. Experimental myositis ossificans: cartilage and bone formation in muscle in response to diffusible bone matrix derived morphogen. Arch Pathol Lab Med 1978;102:312-6. [PubMed]
- Kurer MH, Khoker MA, Dandona P. Human osteoblast stimulation by sera from paraplegic patients with heterotopic ossification. Paraplegia 1992;30:165-8. [PubMed]
- Chantraine A, Minaire P. Para-osteo-arthropathies: a new theory and mode of treatment. Scand J Rehabil Med 1981;13:31-7. [PubMed]
- Freed JH, Hahn H, Menter R, et al. The use of three-phase bone scan in the early diagnosis of heterotopic ossification (HO) and in the evaluation of Didronel therapy. Paraplegia 1982;20:208-16. [PubMed]
- Pakos EE, Ioannidis JP. Radiotherapy vs. Nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys 2004;60:888-95. [Crossref] [PubMed]
- Baird EO, Kang QK. Prophylaxis of heterotopic ossification - an updated review. J Orthop Surg Res 2009;4:12. [Crossref] [PubMed]
- van Kuijk AA, Geurts AC, van Kuppevelt HJ. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord 2002;40:313-26. [Crossref] [PubMed]


