Survival implications of transbronchial cryobiopsy and other diagnostic modalities in idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a relentlessly progressive fibrotic lung disease with a median survival of only 2 to 3 years (1). Approximately half of IPF diagnoses are established by characteristic features on high resolution computerized tomographic scan of the chest (HRCT). When the HRCT is atypical, surgical lung biopsy (SLB) is recommended (2), but carries significant risk of perioperative morbidity and mortality (3). Since Food and Drug Administration (FDA) approval of antifibrotic therapy (nintedanib and pirfenidone) for IPF, there has been renewed interest in pursuing confirmatory histologic diagnosis in patients with suspected IPF (4,5). Transbronchial cryobiopsy (TBC) is a bronchoscopic technique that yields large, high-quality samples of lung tissue without crush artifact in a minimally invasive manner, with a diagnostic yield of 70–80% (6,7). However, there remain concerns about safety of this relatively novel biopsy modality, and there are no existing reports of long-term outcomes of treated IPF following diagnosis by cryobiopsy compared to other diagnostic modalities.
We identified consecutive patients diagnosed with IPF by HRCT, SLB, or TBC in the Vanderbilt University Medical Center (VUMC) clinical interstitial lung disease (ILD) registry who were treated with either pirfenidone or nintedanib between September 19, 2006 and June 1, 2017. This study was approved by the Vanderbilt University Institutional Review Board (IRB# 151099). Since the now FDA approved antifibrotic treatments affect disease progression, we restricted our analysis to subjects treated with pirfenidone or nintedanib to minimize the potential for confounding treatment effect between groups. Subjects in pirfenidone or nintedanib clinical trials randomized to the experimental arm were included. Baseline data including demographics, smoking history, comorbidities, serial pulmonary function testing and diagnostic modality were prospectively collected for all subjects according to the clinical ILD registry protocol and verified by one of the investigators (JK Pannu, AB Smith, WR Mason). Charlson Comorbidity Index was calculated for all subjects at the time of diagnosis. Date of diagnosis was defined as the date of final diagnostic procedure or test establishing the diagnosis of IPF based on current ATS/ERS criteria (2). Vital status was determined at last known date of contact made with our health system as of November 1, 2017. Subjects were categorized into three mutually exclusive groups based on the modality that led to their diagnosis: (I) transbronchial cryobiopsy, (II) surgical lung biopsy, or (III) high resolution computerized tomographic scan of the chest. The primary outcome was adjusted lung-transplant-free survival following the diagnostic test. Continuous variables are reported as mean ± standard deviation or median with interquartile range. Categorical variables are reported as frequencies. Between-group comparisons were conducted using Wilcoxon rank-sum test or Pearson w2 for continuous and categorical variables, respectively. A 2-sided P value of less than 0.05 was considered significant. All analyses were performed using SPSS Statistics v.23 (IBM Corp., Armonk, NY, USA). Survival curves were calculated using Kaplan-Meier method from the time of diagnosis and adjusted for age and comorbidities. Survival times were censored by date of death or lung transplant. Cox regression hazards model was used to assess association between diagnostic techniques and overall survival outcome with the adjustment of age and Charlson Comorbidity Index.
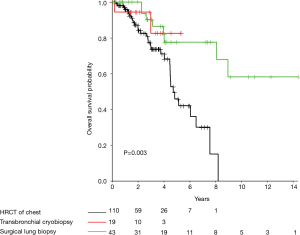
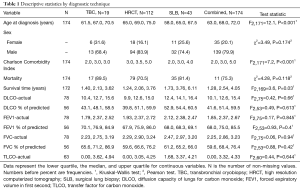
A total of 174 consecutive patients diagnosed with IPF between September 19, 2006 and June 1, 2017 with subsequent antifibrotic treatment were analyzed. IPF was diagnosed by TBC in 19 (10.9%) subjects, SLB in 43 (24.7%), and HRCT in 112 (64.4%). Subjects who underwent biopsy for a diagnosis were significantly younger [HRCT =69 years (65.0–75.0), TBC =67 years (61.5–70.5), SLB =65 years (58–67.5), P<0.001] and had fewer comorbidities (Charlson Comorbidity Index HRCT 3.5 versus 3.0 in SLB and TBC group, P<0.001). Pulmonary function at time of diagnosis did not significantly differ between groups (Table 1). Cox regression analysis adjusted for age and Charlson Comorbidity Index found that diagnosis by SLB was associated with significant longer transplant-free survival than those diagnosed by HRCT [Figure 1; median years survival 3.76 (95% CI, 1.73–6.11) vs. 2.06 (95% CI, 1.24–3.76), P=0.003]. A similar pattern was observed for TBC in comparison to HRCT, however in this small sample, this did not reach statistical significance.
Full table
These data suggest clinically important differences between IPF patients based on HRCT pattern. While most subjects in this analysis had a usual interstitial pneumonia (UIP)-pattern, biopsy was required to diagnose approximately one third of the cohort, consistent with recent trial data (4,5,8). Despite similar disease severity at the time of diagnosis according to pulmonary function testing, treated patients without UIP-pattern on HRCT who thus required biopsy demonstrated significantly better survival after diagnosis (adjusted for age and comorbidities). These results suggest that typical UIP pattern on HRCT may represent a more severe or advanced phenotype of disease (9). Interestingly, contrary to prior report, TBC was not significantly associated with improved survival compared to SLB (7), which may be due in part to the heterogeneity of diagnoses in the previous study; in our cohort, there was no significant difference in survival between TBC and SLB-based diagnoses, although our study had limited power to reveal significant differences. While the number of cases diagnosed by TBC is relatively small in this report, it remains one of the largest studies including IPF patients treated with antifibrotic agents diagnosed with TBC, and to our knowledge the only study directly comparing the long-term outcomes of treated IPF patients using prospectively collected data. This hypothesis-generating result suggests a possible benefit of earlier diagnosis through biopsy before typical radiological findings of IPF become evident and that TBC might be a possible, less invasive alternative to SLB. Most published data on TBC in IPF have been retrospective in nature and limited to immediate procedural and post-procedural morbidity and mortality. Our study suggests that other clinically relevant outcome measures (i.e., transplant-free survival) may be important in determining the utility of this diagnostic modality. Future prospective multicenter studies are needed to better clarify the role of TBC in the diagnosis of IPF and other diffuse parenchymal lung diseases.
Acknowledgements
Funding: This work was supported by NIH K08HL130595 (JA Kropski), Francis Family Foundation (JA Kropski), Pulmonary Fibrosis Foundation (JA Kropski).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ley B, Collard HR, King TE Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Hutchinson JP, Fogarty AW, McKeever TM, et al. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016;193:1161-7. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083-92. [Crossref] [PubMed]
- Griff S, Ammenwerth W, Schönfeld N, et al. Morphometrical analysis of transbronchial cryobiopsies. Diagn Pathol 2011;6:53. [Crossref] [PubMed]
- Lentz RJ, Argento AC, Colby TV, et al. Transbronchial cryobiopsy for diffuse parenchymal lung disease: a state-of-the-art review of procedural techniques, current evidence, and future challenges. J Thorac Dis 2017;9:2186-203. [Crossref] [PubMed]
- Kaarteenaho R. The current position of surgical lung biopsy in the diagnosis of idiopathic pulmonary fibrosis. Respir Res 2013;14:43. [Crossref] [PubMed]
- Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58:143-8. [Crossref] [PubMed]