Trends in lung cancer screening in the United States, 2016–2017
Introduction
Lung cancer kills almost 160,000 people yearly and is responsible for more deaths than breast, colon, prostate and pancreas cancers combined (1). More than 80 percent of patients with lung cancer are diagnosed at an advanced stage (2). The National Lung Screening Trial (NLST), which randomized high-risk patients to receive or not to receive low-dose computed tomography (LDCT) screening, demonstrated a 20 percent reduction in mortality among patients who received LDCT screening (3). Notably, LDCT is the first cancer screening test found to reduce overall mortality, not just cancer-specific mortality. After the results of the NLST were released in 2011, the United States Preventive Services Task Force officially recommended yearly LDCT screening for patients aged 55–77 with at least a 30 pack-year history who had smoked within the last 15 years (4). Soon thereafter, lung screening programs were created at various institutions across the country (5,6). But there is growing concern that the implementation of these programs has not been widespread. Reports using the Behavioral Risk Factor Surveillance System (BRFSS) survey data estimated that fewer than 5% of eligible patients receive LDCT screening (7). Our goals were to determine the frequency and geographic variability of LDCT screening in the United States in an insured population.
Methods
Source of data
After institutional review board approval was obtained from the Institutional Review Board Committee (IRB 17-109), a retrospective cohort study was performed using enrollment and claims data from Clinformatics Data Mart (CDM), one of the nation’s largest commercial health insurance databases with more than 18,000,000 enrollees (8). Data include the Member Eligibility Tables, which contains information on every member enrolled with the health plan during the specific period, and the Medical Claims Tables, which contains data for inpatient and outpatient professional services including outpatient surgery, laboratory, and radiology. The BRFSS data in 2016 was used to estimate the current smoking rate in each state. The University of Texas Medical Branch Institutional Review Board approved the research and waived informed consent.
Cohorts
We developed separate cohorts for 2016 and 2017. Each cohort included all beneficiaries aged 55–77 years on January 1 of that year, with complete insurance enrollment in that year. In analyses that included comorbidity we restricted the cohort to those with coverage for the prior year (n=2,809,801 for 2016 and 3,227,913 for 2017). The steps for the selection of the cohorts are outlined in the Figure S1.
Beneficiary and regional characteristics
Files provided information on beneficiary age, sex and state information. Chronic obstructive pulmonary disease (COPD) or emphysema was identified by ICD-9 codes 490, 491, 492, 496 or ICD-10 codes J41.1, J43.0, J43.1, J43.2, J43.8, J43.9, J44.0, J44.1, or J44.9 associated with inpatient or outpatient billing claims for the previous 12 months. Elixhauser comorbidity measures with COPD and emphysema excluded were generated from all claims in the 12 months before the date of the LDCT and categorized according to number of comorbidities (0, 1, 2, 3, 4+) (9). We also estimated the presence of smoking-related diagnoses in the 12 months prior to LDCT (not including the date of LDCT) defined by the code V15.82 (history of tobacco use), or ICD-9 codes 305.1 (tobacco use disorder) or 989.84 (toxic effect of tobacco).
Outcomes
The primary outcome measured was whether a patient underwent a LDCT (CPT G0297 or S8032).
Statistical analyses
The proportions of beneficiaries receiving LDCT were calculated for each month from January 2015 to December 2017. We then analyzed the time trends in LDCT using joinpoint analysis with a non-linear model to identify change points and 95% confidence intervals, and also slopes between the change points (10). Statistical significance for the joinpoint model analysis was present (P<0.01).
For 2016 and 2017, we calculated the proportions of enrollees stratified by patient characteristics. We estimated the relative risk (RR) of undergoing LDCT using odds ratio from logistic regression (11). Because of the size of the cohorts, the 95% confidence intervals for estimates were small, and small differences were statistically significant. Our focus was more on clinical meaningful differences. The proportions of patients with a charge of LDCT for each state in 2017 were calculated to evaluate the state-level variation. The current daily smoking rate in each state was estimated by all patients aged 55–80 from BRFSS in 2016. The correlation between LDCT and current smoking rates was tested by Spearman rank correlation.
All statistical analysis was performed using SAS/STAT software (SAS Institute Inc. 2008 SAS/STAT 9.2, SAS Institute Inc., Cary, NC, USA).
Results
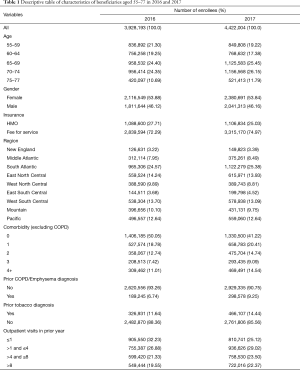
Table 1 shows characteristics of the enrollees, which differed slightly between 2016 and 2017. In 2017, 36.60% were under 65 years old and 53.84% were females; 25.03% were in a health maintenance organization (HMO), an organization in which enrollees pay a fee in return for a range of medical services from providers registered with the organization. Enrollees in 2017 had more comorbidities, a higher rate of prior tobacco diagnoses and more outpatient visits in the previous year. The South Atlantic region was overrepresented compared to the rest of the country.
Full table
Figure 1 shows the rate of LDCT screening for each month in 2016 and 2017. The rate rose throughout 2016 and early 2017, and appeared to plateau by approximately July 2017. Joinpoint analysis detected a significant increase in slope around January 2017, from a slope of 0.2 additional enrollees receiving LDCT per 1,000 enrollees per year prior to January 2017 to a slope of 0.4 enrollees per 1,000 per year between January 2017 and May 2017. Thereafter, there was a decrease from 0.4 enrollees per 1,000 per year to 0.1 enrollees per 1,000 per year.
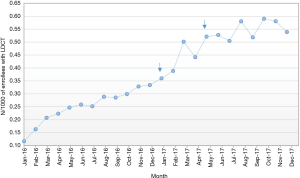
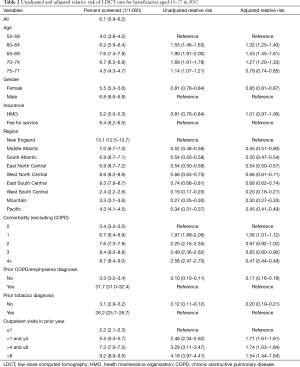
Table 2 shows factors associated with the rate of LDCT screening among enrollees. In the multivariable analyses, enrollees aged 60 to 69 had the highest rates, with those aged 55–59 and 75–77 with the lowest rates. Women had 15% lower odds of receiving LDCT (RR =0.85; 0.81–0.87). There was no difference by whether the enrollee had an HMO vs. fee for service plan, defined as a plan in which medical services which are provided are unbundled and reimbursed separately. Enrollees with 3 or 4+ comorbidities were less likely to receive LDCT. A prior diagnosis of COPD or a diagnosis of current or past tobacco use were both strongly associated with LDCT.
Full table
There was also marked regional variation, with enrollees in the West South Central region only one fifth as likely as those in New England to receive LDCT (RR =0.20; 0.18–0.21). Figure 2 presents a map of the LDCT rates in 2017 by states, with rates varying from 1.1 per 1,000 enrollees per year in Oklahoma to 16.7 per 1,000 enrollees per year in Rhode Island.
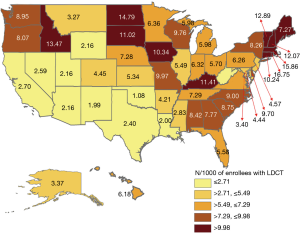
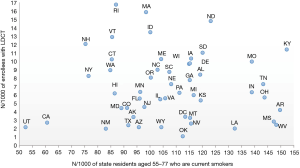
Figure 3 present a scatter plot showing the LDCT rates in each state and the rates of daily smoking among those age 55–79 in each state estimated by 2016 BRFSS data. There was wide variation in both rates, but no correlation between LDCT rate in each state and the daily smoking rates (P=0.87).
Discussion
Lung cancer has a poor overall prognosis, with a 5-year survival of only 18% (12). These poor outcomes occur largely because most diagnoses are made in advanced stages. LDCT screening combats this problem by diagnosing cancers at earlier stages. A lung cancer detected by LDCT screening will be discovered at an early stage 64% to 85% of the time (13,14). When comprehensive LDCT screening is implemented in a community, the rate of stage IV diagnoses for the entire community can drop below 15 percent (15). The benefits of LDCT screening have been demonstrated in numerous trials, but there has been difficulty in establishing effective programs which screen a high percentage of eligible patients (16).
To our knowledge, this current study is the first national study using health insurance claims data to estimate rates of LDCT screening. Prior reported rates of LDCT have utilized survey data, which depend on recall and also on a respondent distinguishing LDCT from other radiologic exams (17). Also, the number of subjects on whom there are data is much higher in claims data, allowing for more precise estimation of changes in LDCT rates over time and geographically.
To get an estimate of the percentage of eligible enrollees who underwent screening, we utilized a study from Jemal and Fedewa (18) which claimed that there were 8.4 million people eligible for LDCT screening in 2010. Using that statistic and United States Census data, 13.2% of adults aged 55 to 77 years nationwide are eligible for LDCT screening. If we accept that estimate and apply it to our data, then approximately 4.6% of eligible patients in our study received a LDCT. Other studies have shown similarly low rates of LDCT screening, and these low rates lag well behind screening rates for colon, prostate and breast cancer. While it might be expected that a new test would initially be underutilized and then gradually become more widespread, our data suggest very little increase in national LDCT rates after May of 2017.
The rate of LDCT screening in each state did not necessarily correlate with the rate of smoking. This was unexpected, because current daily smoking rates by state should correlate strongly with eligibility for LDCT screening. The New England states, which have relatively low rates of smoking, highlighted this discordance. One factor which may account for the high rate of screening in New England is the number of approved lung cancer registry sites by the American College of Radiology (ACR). New England is heavily concentrated with approved sites, compared to the rest of the country (7). Future studies could investigate other possible factors, such as the availability of primary care providers, academic medical centers, socioeconomic and racial/ethnic composition, and other elements which may influence LDCT screening rates.
Our study showed that the youngest and oldest groups of eligible patients—those aged 55–59 and 75–77—had the lowest rates of LDCT screening. It is possible that patients aged 55–59 have not accumulated enough smoking exposure to be eligible. But given that 90% of smokers begin by age 18 or younger (19), it is likely that there is a larger percentage of eligible patients in this range, compared to other ages, who are not being screened. One of the primary reasons for this trend may be a lack of awareness among people within this age group. Campaigns to increase awareness in younger patients should help to increase the percentage of patients overall who receive appropriate LDCT screening. Given that approximately 12,000 patients between the ages of 55 to 59 die each year from lung cancer (20), increasing screening in this age group could have a major impact in reducing overall lung cancer mortality.
We found no difference in LDCT screening rates by type of health plan, HMO vs. fee-for-service. Prior studies have found that patients in HMOs were more likely to receive recommended preventive medicine interventions (21). It may be that enrollees in HMOs have lower rates of eligibility for LDCT screening, which we cannot determine with our data.
The minimal increase in LDCT screening rates after May of 2017 suggests that additional measures are required to increase rates. Lack of awareness can be improved with more advertising promoting the benefits of screening. Increased awareness among patients would explain why patients with more outpatient visits in the prior year were more likely to undergo LDCT screening. Existing screening programs can be made more efficient and employ a multidisciplinary approach. And sharing of electronic medical record between screening programs and local hospitals/physicians will allow for more patients to be included in these screening programs (22).
There were some limitations to this study. The Clinformatics data do not include information on enrollee race/ethnicity. Certain regions such as the South Atlantic are over-represented in the data. The 55–64 age group selects for employed individuals and their families. A major limitation is the lack of information on eligibility for LDCT screening. The only information related to tobacco was the use of tobacco-related diagnoses in the prior year, such as “tobacco use disorder”. Such diagnoses are fairly specific in identifying tobacco use, but with low sensitivity (23). Also, there is no way to determine quantity or duration of smoking. Other criteria for LDCT, such as willingness to undergo surgery if a cancer is found, are also not available in these data.
Our review of enrollees aged 55–77 years in the CDM database revealed that the rate of LDCT screening is low, and is increasing only minimally over time. There are large geographic differences in screening rates which are independent of smoking exposure. The youngest enrollees are least likely to receive screening but may have the most potential to gain quality-adjusted life years. Patients who interact more with physicians are more likely to receive LDCT screening. The marked geographic variation provides an opportunity to study areas with high vs. low LDCT rates to determine factors associated with increased utilization.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The University of Texas Medical Branch Institutional Review Board approved the research and waived informed consent.
References
- National Cancer Institute Surveillance, Epidemiology and End Results Program. Available online: https://seer.cancer.gov/statfacts/html/lungb.html. Accessed August 14th, 2018.
- Gould MK. Clinical Practice. Lung cancer screening with low-dose computed tomography. N Engl J Med 2014;371:1813-20. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Moyer VA. U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the veterans health administration. JAMA Intern Med 2017;177:399-406. [Crossref] [PubMed]
- Armstrong K, Kim JJ, Halm EA, et al. Using lessons from breast, cervical and colorectal cancer screening to inform the development of lung cancer screening programs. Cancer 2016;122:1338-42. [Crossref] [PubMed]
- Charkhchi P, Kolenic GE, Carlos RC. Access to lung cancer screening services: preliminary analysis of geographic service distribution using the ACR lung cancer screening registry. J Am Coll Radiol 2017;14:1388-95. [Crossref] [PubMed]
- Gunaseelan V, Kenney B, Lee JS, et al. Invited commentary: databases for surgical health services research: Clinformatics Data Mart. Surgery 2019;165:669-71. [Crossref] [PubMed]
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8-27. [Crossref] [PubMed]
- Smith PL. Splines as a useful and convenient statistical tool. The American Statistician 1979;33:57-62.
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690-1. [Crossref] [PubMed]
- Hubbard MO, Fu P, Margevicius S, et al. Five-year survival does not equal cure in non-small cell lung cancer: a Surveillance, Epidemiology and End Results-based analysis of variables affecting 10-to-18-year survival. J Thorac Cardiovasc Surg 2012;143:1307-13. [Crossref] [PubMed]
- Kanodra NM, Silvestri GA, Tanner NT. Screening and early detection efforts in lung cancer. Cancer 2015;121:1347-56. [Crossref] [PubMed]
- Deffebach ME, Humphrey L. Lung cancer screening. Surg Clin North Am 2015;95:967-78. [Crossref] [PubMed]
- Okereke IC, Bates MF, Jankowich MD, et al. Effects of implementation of lung cancer screening at one veterans affairs medical center. Chest 2016;150:1023-9. [Crossref] [PubMed]
- Fintelmann FJ, Bernheim A, Digumarthy SR, et al. The 10 pillars of lung cancer screening: rationale and logistics of a lung cancer screening program. Radiographics 2015;35:1893-8. [Crossref] [PubMed]
- Huo J, Shen C, Volk RJ, et al. Use of CT and chest radiography for lung cancer screening before and after publication of screening guidelines: intended and unintended uptake. JAMA Intern Med 2017;177:439-41. [Crossref] [PubMed]
- Jemal A, Fedawa S. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol 2017;3:1278-81. [Crossref] [PubMed]
- U.S. Department of Health and Human Services. The health consequences of smoking-50 years of progress. A report of the surgeon general. Available online: https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf, Accessed August 16, 2018.
- Tanoue LT, Tanner NT, Gould MK, et al. Lung cancer screening. Am J Respir Crit Care Med 2015;191:19-33. [Crossref] [PubMed]
- Xiao Q, Savage GT. HMOs’ consumer-friendliness and preventive health care utilization: exploratory findings from the 2002 Medical Expenditure Panel survey. J Health Hum Serv Adm 2008;31:259-89. [PubMed]
- Raz DJ, Dunham R, Tiep B, et al. Augmented meaningful use criteria to identify patients eligible for lung cancer screening. Ann Thorac Surg 2014;98:996-1002. [Crossref] [PubMed]
- Rostron BL, Chang CM, Pechacek TF. Estimation of cigarette smoking-attributable morbidity in the United States. JAMA Intern Med 2014;174:1922-8. [Crossref] [PubMed]