
Long-term outcomes of multiple and single arterial off-pump coronary artery bypass grafting
Introduction
Coronary artery bypass grafting (CABG) is commonly used to treat left main coronary disease or three-vessel disease with significant efficacy (1). It is the current gold standard to anastomose the left internal mammary artery (LIMA) to the lesioned left anterior descending artery (LAD), while the other lesions are often treated with saphenous vein grafts (SVG) (2). The 10-year patency of LIMA grafts was over 90%, whereas that of venous grafts was only about 50% (3,4). Due to the satisfactory long-term efficacy of single internal mammary artery graft (SIMA) (5), surgeons try to complete CABG with multiple arterial (MA) grafts, especially with bilateral internal mammary artery (BIMA) grafts (6). Several meta-analyses have shown that the 10-year all-cause mortality in the BIMA group was approximately 20% lower than that in the SIMA group (7-11). An observational study showed the improved survival with radial artery (RA) versus vein conduits in CABG with LIMA-LAD grafting (12). The result of the Radial Artery Database International Alliance (RADIAL) project showed decreased main adverse cardiovascular and cerebrovascular events (MACCE) rate with RA versus SVG (13).
Additionally, the current US and European guidelines encourage the use of arterial grafts (AGs) in patients with long life expectancies (14,15). However, the current evidence on the clinical effects of using multiple versus single AG for CABG is still controversial. The ten-year result of Arterial Revascularization Trial (ART), an international multi-center randomized controlled trial (RCT) enrolling more than 3,000 patients to compare BIMA with SIMA, was released in ESC2018. It showed no survival benefit of BIMA. A recent pooled analysis of individual patient data from the six RCTs comparing the RA to the SVG found no significant reduction of follow-up death 5 years after surgery (13).
Meanwhile, it is worth noting that the use of AGs remains limited all around the world. Currently, in the USA, less than 6% of all CABG patients receive more than 1 AG (16), while the rate of BIMA use is slightly above 12% in Australia (17). A possible explanation for the slow diffusion of the use of AGs is the lack of substantial evidence concerning its clinical benefits. Off-pump coronary artery bypass grafting (OPCAB) is a safe and effective operation for treating multivessel disease with multiple AGs (18).
Most articles about MA CABG had strict limitations for the type of AGs, and mostly compared LIMA + RA with LIMA + SVG or BIMA with SIMA. However, the mid-term patency rate of the RA and right internal mammary artery (RIMA) was similar in a previous study (19). Thus, it may be that the number of AG matters, but not the type of AG. In this study, the patients were divided according to the number of AGs. The purpose of this study was to find whether, in patients undergoing primary isolated non-emergent OPCAB, the use of 2 or more AGs compared with a single AG is associated with a reduction in the long-term adverse events.
Methods
Study design
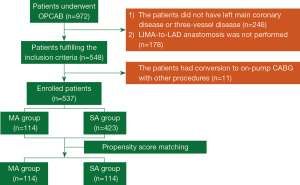
The local Research Ethics Board of Ruijin Hospital approved this retrospective cohort study (No. 2018-40). The local Research Ethics Board waived the written patient consent. Data were collected from the local database of Ruijin Hospital. The patients between January 2006 and December 2008 were selected for analysis. The inclusion criteria were as follows: (I) the patient underwent OPCAB; (II) the patient had left main coronary disease or three-vessel disease; (III) LIMA-to-LAD anastomosis was performed. The exclusion criteria were as follows: (I) the operation was emergent; (II) the incision was not median sternotomy; (III) the patient had a conversion to on-pump CABG with other procedures, such as valve repair; (IV) the OPCAB was a redo surgery.
The patients were divided into the MA group and the single arterial (SA) group according to the number of AGs. The patients who had at least 2 AGs, one of which was LIMA-LAD were included into the MA group. For example, the patients with LIMA + RA, LIMA + RIMA, LIMA + RA + SVG or LIMA + RIMA + RA + SVG were all in the MA group. The SA group only included the patients with LIMA + SVG. Figure 1 shows the detailed flow of this study.
Surgical procedure
The cardiac stabilizer was used to locally secure and fully expose the anastomotic site. A shunt of appropriate size was used to protect the distal myocardial blood supply. An aerosol spray device was used to keep the anastomotic site clear of blood. The proximal anastomosis to the aorta used a 5-0 prolene suturing with an aortic side-biting clamp. The epicardial tissue on the anterior wall of aorta should be removed, and the hole puncher was used for punching a hole on the aorta. After the anastomosis completed, the air was discharged by water flush or blood flow before knotting. The distal anastomosis to the target vessels used 7-0 prolene.
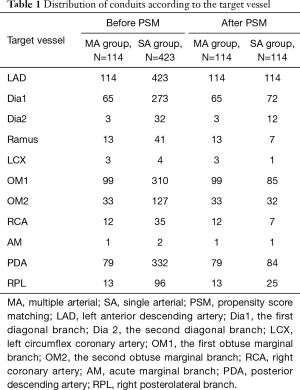
A total of 537 LIMA grafts were involved in this study and anastomosed to LAD in situ. Twelve cases were treated with RIMA, 3 of which were free. The proximal ends were anastomosed to LIMA with end-to-side anastomosis and the distal to OM1 (n=3). The other RIMA grafts were anastomosed to Dia1 (n=8) and RCA (n=1) in situ. A total of 108 cases were treated with RA grafts. The proximal end of the RA graft was anastomosed to LIMA (n=21) and the aorta (n=84) and SVG (n=3) with end-to-side anastomosis. The RA graft was then sequentially anastomosed to the targeted vessels. Only 3 patients used the gastroepiploic arteries (GEA). The GEA grafts were all anastomosed to PDA in situ. SVG was anastomosed with sequential anastomosis or using the “Y/T” anastomosis techniques. The distribution of the conduits according to the target vessels is shown in Table 1.
Full table
All patients received 100 mg of aspirin and 75 mg clopidogrel daily in the first year following the surgery. Then, 100 mg of daily aspirin was continued for life.
Data collection
The preoperative characteristics and the postoperative data, including rates of death, IABP support, the volume of drainage, and length of stay (LOS) in the intensive care unit (ICU), were obtained from the local database of Ruijin Hospital.
Follow-up
Follow-up was completed via a telephone interview annually from our unit. For the patients in this study, an additional telephone interview was performed. The incidence of death, cardiac death, myocardial infarction (MI), fatal MI, stroke, readmission for heart failure and target vessel revascularization (TVR) were recorded. The MACCE was defined as a composite outcome of death, MI and stroke. The primary outcome was cardiac death.
Propensity score matching (PSM)
A total of 114 patients in the MA group and 423 patients in the SA group fulfilled the inclusion and exclusion criteria. To adjust for significant unbalanced individual characteristics between multiple and SA OPCAB patients, we used propensity scores (PSs) to reduce this imbalance (17). A multivariate logistic regression model was employed to estimate PSs using all of the preoperative characteristics. We chose caliper matching without a replacement for this study. A subset of the SA group was matched to the MA group with calipers a width of 0.2 the standard deviation of the logit of the PS. Covariate balance was measured using the standardized mean difference (SMD). An SMD between −10% and 10% indicated a well-matching balance.
Statistical analysis
Continuous variables were summarized as mean ± SD (standard deviation) or median (the 25th percentile, the 75th percentile) and categorical variables were categorized as frequencies and percentages. After PSM, for early outcomes, continuous variables were compared using paired t-tests or Wilcoxon signed-rank test, and categorical variables were compared using McNemar’s test. In the other situations, continuous variables were compared using a two-tailed Student’s t-test. Categorical variables were compared using the χ2 test or Fisher exact test. The time to the first occurrence of any one of the long-term outcomes was described by Kaplan-Meier curves (K-M curves), and the comparisons of K-M curves were performed with a stratified log-rank analysis. A Cox proportional hazards model was used to calculate the unadjusted hazard ratio (HR), and the HR was adjusted via the PS for both data sets relating to all-cause and cardiovascular death for the MA group compared with the SA group. All analysis was performed with SPSS version 22.0 (Chicago, IL, USA) and R version 3.4.3. A P value of <0.05 was considered to be statistically significant.
Results
Baseline characteristics of patients
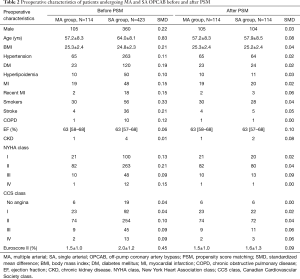
A total of 114 patients in the MA group and 423 patients in the SA group fulfilled the inclusion and exclusion criteria, and there were 114 patients in each group after PSM. All SMDs were less than 0.10, representing negligible differences across all of the preoperative characteristics. Table 2 displays the preoperative patient characteristics before and after PSM.
Full table
Furthermore, there were no differences between the numbers of grafts for the two groups before (3.82±0.91 vs. 3.96±0.94, P=0.156) and after (3.82±0.91 vs. 3.86±0.92, P=0.601) PSM.
Postoperative outcomes
Table 3 shows the postoperative results of the two groups. The mortality, the incidence of IABP support, and the volume of drainage were comparable between the two groups before and after PSM (P>0.05). The LOS in ICU was significantly shorter in the MA group, compared with the SA group either before or after PSM (P=0.001).

Full table
Long-term outcomes
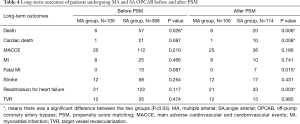
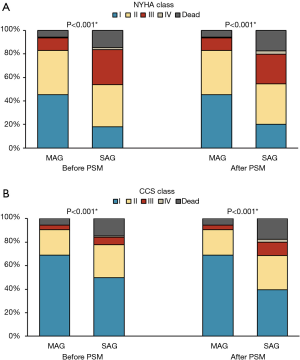
The median follow-up time was 117 months (interquartile range, 110 to 128 months). The follow-up rates of the MA and SA groups were 93.0% and 91.7% respectively, both before PSM (93.0%), and after PSM (100%). Before PSM, the incidences of cardiac death, MACCE, MI, fatal MI, stroke, readmission for heart failure, and TVR were comparable between the two groups (P>0.05), but the mortality of the MA group was significantly lower than that of the SA group (P=0.026) (Table 4). After PSM, there was no difference in the incidences of MACCE, MI, stroke, and TVR between the two groups (P>0.05). However, the frequency of death (P=0.006), cardiac death (P=0.008), fatal MI (P=0.015) and readmission for heart failure (P=0.003) were all significantly lower in the MA group when it was compared with the SA group (Table 4). The distributions of NYHA class (P<0.001) and CCS class (P<0.001) were better in the MA group than in the SA group either before or after PSM (Figure 2A,B).
Full table
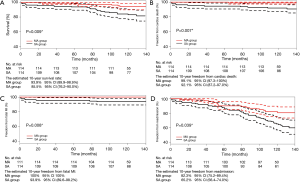
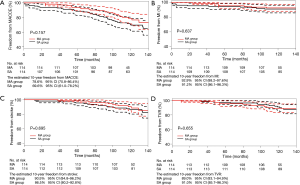
Long-term freedom from adverse events was estimated for each group using Kaplan-Meier analysis after PSM. The ten-year freedom from death, cardiac death, fatal MI and readmission for heart failure were 93.9% vs. 85.5% (P=0.009), 99.1% vs. 92.1% (P=0.007), 100% vs. 93.9% (P=0.008) and 82.3% vs. 65.2% (P=0.039) in the MA group and in the SA group, respectively (Figure 3A,B,C,D). The ten-year freedom from MACCE, MI, stroke, and TVR were not significantly different between the two groups (P>0.05) (Figure 4A,B,C,D).
The univariate Cox proportional hazard model demonstrated that MAOPCAB was significantly associated with decreased risks for all causes of death (HR =0.358, 95% CI: 0.280 to 0.458) and cardiac death (HR =0.167, 95% CI: 0.071 to 0.392). After being adjusted for PS, MAOPCAB were found to be significant determinants for all-cause death (HR =0.546, 95% CI: 0.357 to 0.835) and cardiac death (HR =0.291, 95% CI: 0.104 to 0.818).
Discussion
In recent years, OPCAB and the application of multiple artery grafts have gained more and more attention. The 5-year results of the CORONARY study showed that OPCAB had no significant effect on the incidence of MACCE when it was compared with an on-pump CABG, which meant OPCAB was as safe as the conventional procedure (20). Meanwhile, OPCAB can avoid the microthrombus hypoperfusion and systemic inflammation caused by CPB, which reduces patient injury and is conducive to postoperative recovery. The use of multiple artery grafts offers long-term survival benefits for patients who are under the age of 60 (21), male (22), with DM (23) or with a BMI under 30 (24), compared the use of single artery graft. The American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guidelines for CABG recommended the use of multiple artery grafts to treat patients under 60 years of age with severe coronary artery stenosis (≥90% in the right coronary artery, ≥70% in the left coronary artery) (14). This study attempted to investigate the long-term clinical benefit of MA OPCAB in treating left main coronary disease or three-vessel disease, compared with SA OPCAB.
The postoperative outcomes of MA CABG were satisfactory in previous literature. A pilot RCT showed that no patients who underwent MA or SA on-pump CABG died in hospital, and the incidences of postoperative MI and stroke were comparable between the two groups (25). In this study, there were no patients that died in the MA group in hospital, which was similar to the previous results of MA on-pump CABG, and there were no differences in the postoperative mortality, the incidence of IABP support and the volume of drainage between the two groups. However, the LOS in ICU was significantly shorter in the MA group than in the SA group (P=0.001). These results showed that MA OPCAB was as safe as either SA OPCAB or MA on-pump CABG and had better postoperative recovery compared with SA OPCAB.
For the comparison between SIMA and BIMA, the use of BIMA was associated with a significantly better long-term survival with a relative risk reduction of approximately 20% (26). A meta-analysis including 89,399 patients (27), reported that 8.6 years after surgery, the BIMA cohort had significantly better long-term survival, MI-free, and angina-free survival. Similar benefits have been achieved using an RA as a second AG in conjunction with a SIMA. At a mean follow up of 6.7 years, the use of the RA was associated with a 24% relative decrease in mortality, whereas operative mortality and morbidity were similar (28). Two independent meta-analyses have also found that the use of a third AG and total arterial revascularization are associated with a significant long-term survival benefit (29,30). The explanation for the benefit of multiple AGs is the better patency rate of the AGs compared to SVG. The use of the RA was associated with a 69% relative reduction in the risk of graft failure beyond 4 years of follow-up (31). For the RIMA, there were similar results (32). A network meta-analysis comparing the RA, RIMA, and SVG as the second conduit for CABG found that the SVG was associated with a significantly higher graft occlusion rate at a follow-up exceeding 4 years (19). Another explanation is that AGs can exert a protective effect on the coronary circulation, due to the downstream release of endothelial-derived antithrombotic and anti-inflammatory mediators (33).
However, the current evidence on MACABG is still controversial. The 5-year results (34) and 10-year results released in ESC2018 of ART, the largest RCT comparing BIMA and SIMA, showed no difference in survival and event-free survival between the two groups. After many years of observational evidence actively supporting the better clinical results of BIMA, the result of ART was a surprise for the cardiovascular community. A recent pooled analysis of individual patient data from the six RCTs with a mean follow-up of more than 2 years found a statistically significant reduction in the combined end-point of follow-up cardiac events, but not of follow-up death 5 years after surgery (13).
The mean follow-up time of this study was about 10 years, and the follow-up rate was above 90%, which means the results are high-qualified and may reflect the long-term effect of MA OPCAB. In this study, the incidences of MI were not different between two groups, but MA OPCAB was shown to significantly decrease the long-term impact of fatal MI, compared with SA OPCAB (P=0.015), perhaps resulting in the lower cardiac death rate (P=0.008) and mortality (P=0.006). The results of Kaplan-Meier analysis were consistent with the above results. MA OPCAB significantly improved the ten-year freedom of cardiac death (P=0.007) and death (P=0.009) by increasing the ten-year freedom from fatal MI (P=0.008), compared with SA OPCAB. The better distributions of NYHA class (P<0.001) and CCS class (P<0.001) in the MA group showed that MA OPCAB could lead to symptom improvement in promoting heart function and lowering angina, resulting in the lower readmission rate for heart failure (P=0.003), compared with SA OPCAB.
In conclusion, this study with a 10-year follow-up showed that MA OPCAB was a safe procedure in treating left main coronary disease or three-vessel disease with better postoperative recovery compared with SA OPCAB. MA OPCAB could significantly decrease the long-term incidence of fatal MI, compared with SA OPCAB, resulting in the lower cardiac death rate and mortality. Additionally, MA OPCAB could promote heart function and lower angina, leading to the lower readmission rate for heart failure in comparison with SA OPCAB. This study did not limit the type of AGs anastomosed to non-LAD lesions. Provided the LIMA-LAD anastomosis is completed, the use of 2 or more AGs, whatever the type, compared with a SAG is associated with a reduction in the long-term adverse events.
Limitations
Firstly, this is a nonrandomized comparison. The potential limitation of the study is that there still may be residual confounders, as well as differences between groups due to the nonblinding of both the patients and physicians. Secondly, the sample size was small, and we will design a study which includes more patients in the future.
Acknowledgements
Funding: This paper is funded by National Natural Science Foundation of China (81671832), National Natural Science Foundation of China (81571826) and “Two-hundred Talent” team, Medical School, Shanghai Jiao Tong University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local Research Ethics Board of Ruijin Hospital (No. 2018-40). The local Research Ethics Board waived the written patient consent.
References
- Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563-70. [Crossref] [PubMed]
- Aldea GS, Bakaeen FG, Pal J, et al. The Society of Thoracic Surgeons Clinical Practice Guidelines on Arterial Conduits for Coronary Artery Bypass Grafting. Ann Thorac Surg 2016;101:801-9. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [Crossref] [PubMed]
- Gansera B, Schmidtler F, Angelis I, et al. Patency of internal thoracic artery compared to vein grafts - postoperative angiographic findings in 1189 symptomatic patients in 12 years. Thorac Cardiovasc Surg 2007;55:412-7. [Crossref] [PubMed]
- Hlatky MA, Shilane D, Boothroyd DB, et al. The effect of internal thoracic artery grafts on long-term clinical outcomes after coronary bypass surgery. J Thorac Cardiovasc Surg 2011;142:829-35. [Crossref] [PubMed]
- Glineur D, Papadatos S, Grau JB, et al. Complete myocardial revascularization using only bilateral internal thoracic arteries provides a low-risk and durable 10-year clinical outcome. Eur J Cardiothorac Surg 2016;50:735-41. [Crossref] [PubMed]
- Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001;358:870-5. [Crossref] [PubMed]
- Takagi H, Goto SN, Watanabe T, et al. A meta-analysis of adjusted hazard ratios from 20 observational studies of bilateral versus single internal thoracic artery coronary artery bypass grafting. J Thorac Cardiovasc Surg 2014;148:1282-90. [Crossref] [PubMed]
- Yi G, Shine B, Rehman SM, et al. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Rizzoli G, Schiavon L, Bellini P. Does the use of bilateral internal mammary artery (IMA) grafts provide incremental benefit relative to the use of a single IMA graft? A meta-analysis approach. Eur J Cardiothorac Surg 2002;22:781-6. [Crossref] [PubMed]
- Weiss AJ, Zhao S, Tian DH, et al. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann Cardiothorac Surg 2013;2:390-400. [PubMed]
- Tranbaugh RF, Dimitrova KR, Friedmann P, et al. Radial artery conduits improve long-term survival after coronary artery bypass grafting. Ann Thorac Surg 2010;90:1165-72. [Crossref] [PubMed]
- Gaudino M, Benedetto U, Fremes S, et al. Radial-Artery or Saphenous-Vein Grafts in Coronary-Artery Bypass Surgery. N Engl J Med 2018;378:2069-77. [Crossref] [PubMed]
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;58:e123-210. [Crossref] [PubMed]
- Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517-92. [Crossref] [PubMed]
- ElBardissi AW, Aranki SF, Sheng S, et al. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg 2012;143:273-81. [Crossref] [PubMed]
- Yan BP, Clark DJ, Buxton B, et al. Clinical characteristics and early mortality of patients undergoing coronary artery bypass grafting compared to percutaneous coronary intervention: insights from the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) and the Melbourne Interventional Group (MIG) Registries. Heart Lung Circ 2009;18:184-90. [Crossref] [PubMed]
- Davierwala PM, Leontyev S, Garbade J, et al. Off-pump coronary artery bypass surgery with bilateral internal thoracic arteries: the Leipzig experience. Ann Cardiothorac Surg 2018;7:483-91. [Crossref] [PubMed]
- Benedetto U, Raja SG, Albanese A, et al. Searching for the second best graft for coronary artery bypass surgery: a network meta-analysis of randomized controlled trialsdagger. Eur J Cardiothorac Surg 2015;47:59-65; discussion 65. [Crossref] [PubMed]
- Lamy AR, Devereaux PJ, Yusuf S. Five-Year Outcomes after Off-Pump or On-Pump Coronary-Artery Bypass Grafting. N Engl J Med 2017;376:894-5. [Crossref] [PubMed]
- Benedetto U, Codispoti M. Age cutoff for the loss of survival benefit from use of radial artery in coronary artery bypass grafting. J Thorac Cardiovasc Surg 2013;146:1078-84; discussion 1084-5. [Crossref] [PubMed]
- Pullan M, Kirmani BH, Conley T, et al. The effect of patient sex on survival in patients undergoing isolated coronary artery bypass surgery receiving a radial artery. Eur J Cardiothorac Surg 2015;47:324-30. [Crossref] [PubMed]
- Deb S, Singh SK, Moussa F, et al. The long-term impact of diabetes on graft patency after coronary artery bypass grafting surgery: a substudy of the multicenter Radial Artery Patency Study. J Thorac Cardiovasc Surg 2014;148:1246-53; discussion 1253. [Crossref] [PubMed]
- Benedetto U, Caputo M, Zakkar M, et al. The effect of obesity on survival in patients undergoing coronary artery bypass graft surgery who receive a radial artery. Eur J Cardiothorac Surg 2017;51:376-81. [PubMed]
- Le J, Baskett RJ, Buth KJ, et al. A pilot randomized controlled trial comparing CABG surgery performed with total arterial grafts or without. J Cardiothorac Surg 2015;10:1. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Rahouma M, et al. Unmeasured Confounders in Observational Studies Comparing Bilateral Versus Single Internal Thoracic Artery for Coronary Artery Bypass Grafting: A Meta-Analysis. J Am Heart Assoc 2018.7. [PubMed]
- Buttar SN, Yan TD, Taggart DP, et al. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: a meta-analysis. Heart 2017;103:1419-26. [Crossref] [PubMed]
- Gaudino M, Lorusso R, Rahouma M, et al. A meta-analysis of the randomized and adjusted observational studies comparing the clinical outcome of the radial artery and the saphenous vein as the second conduit for coronary artery bypass surgery. J Thorac Cardiovasc Surg 2019;8:e010839.
- Gaudino M, Puskas JD, Di Franco A, et al. Three Arterial Grafts Improve Late Survival: A Meta-Analysis of Propensity-Matched Studies. Circulation 2017;135:1036-44. [Crossref] [PubMed]
- Yanagawa B, Verma S, Mazine A, et al. Impact of total arterial revascularization on long term survival: A systematic review and meta-analysis of 130,305 patients. Int J Cardiol 2017;233:29-36. [Crossref] [PubMed]
- Cao C, Manganas C, Horton M, et al. Angiographic outcomes of radial artery versus saphenous vein in coronary artery bypass graft surgery: a meta-analysis of randomized controlled trials. J Thorac Cardiovasc Surg 2013;146:255-61. [Crossref] [PubMed]
- Gaudino M, Mack MJ, Taggart DP. Additional Arterial Conduits in Coronary Artery Bypass Surgery: Finally Coming of Age. J Am Coll Cardiol 2018;71:2974-6. [Crossref] [PubMed]
- Fortier JH, Ferrari G, Glineur D, et al. Implications of coronary artery bypass grafting and percutaneous coronary intervention on disease progression and the resulting changes to the physiology and pathology of the native coronary arteries. Eur J Cardiothorac Surg 2018;54:809-16. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]


