
Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: comparison with computed tomography (CT) guided needle biopsy
Introduction
Thoracic malignant diseases including lung cancer or malignant mesothelioma are increasing worldwide and are a leading cause of cancer-related deaths, particularly in Asian countries (1,2). Extensive local invasiveness and the frequency of distant metastases contribute to the poor prognosis in lung cancer. Recent advances in molecular targeted therapy in lung cancer based on epidermal growth factor receptor, anaplastic lymphoma kinase, c-ros oncogene 1, and programmed cell death 1 inhibitor treatments require more sample volume to evaluate targeted histological diagnosis (3,4). Therefore, a more precise diagnostic procedure is necessary for use in determining appropriate management of thoracic malignancy.
Diagnostic modalities for thoracic lesions include surgical biopsy, bronchoscopy, and percutaneous needle biopsy under computed tomography (CT) or ultrasound (US) guidance. Surgical biopsy is a reliable method for diagnosing malignant disease and obtaining an adequate amount of tissue; however, it is invasive and requires general anesthesia. Bronchoscopy is a safe diagnostic modality; however, it has a low diagnostic yield for peripheral lesions, especially when the lesions are located adjacent to the chest wall or within 10 mm from the costal visceral pleura (5).
Percutaneous needle biopsy under imaging guidance (i.e., CT and US) is an effective diagnostic method and less invasive than a surgical biopsy. CT-guided percutaneous needle biopsy for peripheral lung or pleural lesions is particularly well established (6,7), and is commonly performed in clinical settings. Although CT-guided needle biopsy is generally a reliable procedure with a high diagnostic rate (77–96%) (8), it is associated with a high complication rate in addition to radiation exposure; post-procedural pneumothorax is reported to occur in 17–26.6% of cases and hemoptysis in 4–27%. Moreover, pneumothorax after CT-guided biopsy sometimes requires drainage, and hemoptysis requires intubation or blood transfusion (9,10).
Previous studies reported that percutaneous needle biopsy under US guidance was an accurate and inexpensive technique with a short examination time, and allowed real-time monitoring at the bedside (11,12). US-guided percutaneous needle biopsy for thoracic lesions may have several advantages over the CT-guided procedure, with no radiation exposure, a short examination time at the bedside, real-time monitoring of needle placement in the target lesion, and avoidance of vessels through color Doppler imaging. Therefore, US-guided biopsy for eligible cases (such as a lesion directly in contact with the chest wall without an intervening aerated lung) has the potential to be a feasible and reliable biopsy technique as an alternative to CT-guided biopsy.
However, few studies have addressed the efficacy and safety of US-guided biopsy for thoracic lesions. Moreover, it is not yet established whether US- or CT-guided biopsy is preferred in a given case. Therefore, this study evaluated the efficacy and safety of US-guided versus that of CT-guided biopsy in patients with peripheral lung or pleural lesions adjacent to the chest wall.
Methods
Patient selection and study subjects
We retrospectively enrolled and reviewed consecutive patients who underwent US- or CT-guided percutaneous biopsy for peripheral lung or pleural lesions adjacent to the chest wall at Osaka City University Hospital between April 2012 and December 2017. A repeat biopsy performed at a different time in the same patient was treated as a different case. We excluded biopsies performed for thoracic lesions that were not adjacent to the chest wall. Written informed consent was obtained from all patients for the biopsy, in accordance with the ethical standards of the World Medical Association (Declaration of Helsinki). This study was approved by the Ethics Committee of our institution (#1700 and #3433). Patients who needed thoracic percutaneous needle biopsy for definitive diagnosis were identified by each respiratory physician or a respiratory medicine team case conference and asked to undergo US- or CT-guided biopsy without specific criteria.
Clinical characteristics including age, sex, body mass index (BMI), lesion location, the presence of a pleural effusion, lesion size, lesion-pleura contact arc length (LPCAL), sample number obtained, and pathological diagnosis were reviewed. Lesion location was classified in 4 areas along 4 main anatomical lines (midclavicular, anterior axillary area, posterior axillary, and paravertebral areas). LPCAL was measured as described previously (13) (Figure 1). The diagnostic rate and post-procedural complications were compared between the US- and CT-guided groups.
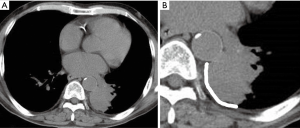
Biopsy procedures
CT-guided percutaneous needle biopsy
CT-guided biopsies were performed by 3 experienced respiratory physicians (each with at least 5 years of experience and more than 5 years after completing a Japanese senior residency) using a procedure similar to that previously reported (14). Briefly, thoracic lesions were imaged on CT (CT-W2000AD, Hitachi, Tokyo, Japan) as 5-mm-thick contiguous axial tomographic sections, at 120 kVp and 100 mA, before CT-guided needle biopsy. After lesions were detected, preliminary helical CT images were obtained in 3-mm-thick sections through the lesion. Following administration of local anesthesia containing 1% lidocaine and insertion of an 18-G introducer needle (needle length, 100 mm; Hakko, Tokyo, Japan), biopsies were performed with a 19-G core tissue biopsy needle Bird®Magnum® (Bard, Covington, LA, USA). After the procedure, chest CT images were obtained to detect any complication such as pneumothorax or hemorrhage. Patients were continuously monitored with oximetry, electrocardiography, and blood pressure measurement after the procedure while remaining in a supine or prone position for at least 2 hours. Chest X-ray was also obtained.
US-guided percutaneous needle biopsy
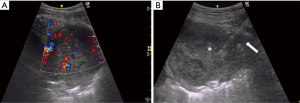
US-guided biopsies were performed by 3 experienced respiratory physicians (each with at least 3 years of experience and more than 5 years after completing a Japanese senior residency) using an US system (GE Healthcare LOGIQ e) at the bedside in the respiratory ward. Patient position (supine or prone) was decided in advance based on the location of the lesion using the shortest and safest approach to visualize movement of the US probe. For each US-guided biopsy, operators chose a 4C or 12L transducer. After local antisepsis and anesthesia, the biopsy needle was inserted using a sterile puncture transducer with a needle-guide attachment and variable angle selection through a lateral or medial approach. Real-time color Doppler imaging was used to avoid vessels (Figure 2). The biopsy needle was advanced toward the lesion with real-time imaging using an 18-G needle, Bird®Monopty® (Bard). After the procedure, chest X-ray was obtained to identify any complications. The patient was monitored for 2 hours.
Diagnostic categories
Pathological findings in biopsy samples were divided into 3 categories: “malignancy”, “others”, and “no diagnosis”. Malignancy included any malignant diseases.
Others included benign tumors, organizing pneumonia, and other non-malignant diseases. No diagnosis included insufficient tissue and necrosis. The findings of malignancy and others were considered successful biopsies in the statistical analysis.
Statistical analysis
The comparisons of the 2 groups were evaluated with Fisher’s exact test for categorical values and the Mann-Whitney U test for numerical values, and P<0.05 was considered significant. Logistic regression was used for univariate and multivariate analyses to identify the factors affecting diagnostic rates. Our multivariable models included examination procedure (US, CT), age (≥75, <75 years), sex (male, female), BMI (≥22, <22 kg/m2), lesion location (anterior, midclavicular to anterior axillary, posterior, and posterior axillary to paravertebral areas), post-procedural complications (yes, no), and the presence of a pleural effusion (yes, no). Statistical analyses were performed using JMP10 software (SAS Institute, Inc., Cary, NC, USA).
Results
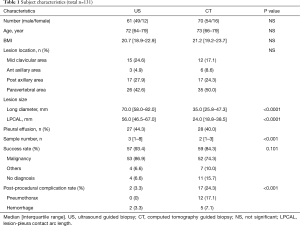
We enrolled a total of 131 biopsy cases (US-guided; n=61, and CT-guided; n=70) for thoracic lesions adjacent to the chest wall. Two patients in the CT-guided group were excluded from this study because of cancellation of the biopsy procedure due to pain and respiratory failure. One patient in the US-guided group was excluded because of cancellation of the procedure due to poor imaging under US guidance. CT-guided biopsy was performed in that patient at a different time. The characteristics of the 2 biopsy procedure groups are shown in Table 1.
Full table
No significant difference was found between the 2 groups in age, sex, BMI, lesion location, and the presence of a pleural effusion. Lesion size and LPCAL in the US-guided group were significantly greater than those in the CT-guided group (P<0.0001). The US-guided group yielded a significantly larger number of samples than the CT-guided group (P<0001).
Among US-guided biopsies, 53 samples were categorized as malignancies: 40 non-small cell lung cancers, 5 malignant lymphomas, 4 small cell lung cancers, 2 metastatic lung cancers, 1 malignant mesothelioma, and 1 sarcoma. Four biopsy samples were categorized as others: 2 thymomas, 1 organizing pneumonia, and 1 lipoma. The last 4 biopsy samples were categorized as no diagnosis: 2 non-malignant and 2 necrosis.
Among CT-guided biopsies, 52 samples were categorized as malignancies: 40 non-small cell lung cancers, 3 small cell lung cancers, 7 metastatic lung cancers, 1 malignant mesothelioma, and 1 malignant thymic cancer. Seven biopsy samples were categorized as others: 2 organizing pneumonias, 2 granulation tissues, 1 tuberculoma, 1 schwannoma, and 1 methotrexate-associated lymphoproliferative disorder. The last 11 biopsy samples were categorized as no diagnosis: 8 no malignancy specimens, 2 necrosis, and 1 insufficient material.
The biopsy success rate was marginally but not significantly higher in the US-guided group (93.4%) than in the CT-guided group (84.3%) (P=0.101). The median value of LPCAL was 40 mm. When data were divided into large/small LPCAL groups (median cut-off in all participants =40 mm; large: ≥40 mm; small: <40 mm), the diagnostic rate for lesions larger than 40 mm in the US-guided group was significantly higher than in the CT-guided group (P=0.009) (Table 2).

Full table
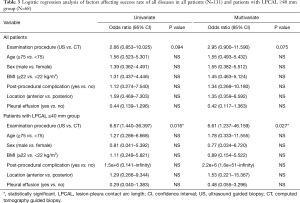
Univariate and multivariate analyses identified no variables that affected the biopsy success rate in all populations. However, the examination procedure was significantly affected by the biopsy success rate in patients with LPCAL ≥40 mm (odds ratio for US-guided vs. CT-guided: univariate 0.15, multivariate 0.12 for all diseases) (Table 3).
Full table
Total post-procedural complications (3.3%; 0 pneumothorax, 2 hemorrhages) in the US-guided group were significantly lower than in the CT-guided group (24.3%; 12 pneumothorax, 17.1%; 5 hemorrhages, 7.1%). None of the patients in the US-guided group had detectable post-procedural pneumothorax or hemorrhage requiring treatment. In the CT-guided group, 3 (4.3%) patients required post-procedural treatment via chest tube placement (n=2) or surgery (n=1).
Discussion
The current study confirmed the acceptable efficacy and safety of CT-guided biopsy for thoracic lesions and revealed that US-guided biopsy had a higher diagnostic rate with a longer LPCAL and a higher success rate without complications. The US-guided group also yielded a significantly larger number of samples than the CT-guided group (P<0001), suggesting that US-guided biopsy might be safer for repeat biopsy than CT-guided biopsy. US-guided biopsy for thoracic lesions adjacent to the chest wall may be a feasible technique with respect to efficacy and safety compared with CT-guided biopsy.
Although bronchoscopy is a safe modality, the reported diagnostic yield for peripheral lesions using radial endobronchial US and a guide sheath is comparatively low at 55%, while the diagnostic yield for central parenchymal lesions is 77% (5). The success rates for US- and CT-guided biopsies in this study were 93.4% and 84.3%, respectively. These rates were acceptable compared with those reported in previous studies (US-guided: 84–96%, CT-guided: 77–96%) (9,11,15-18). In our study, the lesion size and LPCAL in the US-guided group were significantly greater than those in the CT-guided group (P<0.0001). Most respiratory physicians consider a small lesion difficult to reach with US-guided biopsy. However, Jarmakani et al. recently reported that there was no correlation between small lesion size and diagnostic accuracy in both US- and CT-guided biopsies. They also reported a free-hand approach in US procedures, which provides more flexibility to reach even smaller lesions (<1.5 cm) (11). Other studies have shown that diagnostic accuracy generally decreases with decreasing lesion size, e.g., a solitary peripheral lung nodule, even with CT-guided biopsy (19,20).
Surprisingly, US-guided biopsy showed high diagnostic accuracy in patients with bulky lesions in greater contact with the chest wall. In contrast, the diagnostic rate of large mass lesions was comparatively decreased with CT-guided biopsy. Jeon et al. also reported that among 97 US-guided biopsies for lesions contacting the pleura, the “pleural contact length” significantly influenced diagnostic accuracy (13). Possible reasons are as follows: In large lesions, such as squamous cell lung carcinoma, central necrosis is often present, resulting in necrotic or inadequate samples. Needle insertion and biopsy under CT guidance are performed blindly, while needle placement can be performed with real-time visualization of the lesion under US guidance. Moreover, repeatability and a larger number of samples may increase the success rate. The approach of various angles to the lesion and visualization of the necrotic area and solid tumor based on sonographic features is also an advantage of US-guided biopsy (21).
Our US-guided procedure in patients with LPCAL ≥40 mm was significantly affected by the biopsy success rate. This finding may suggest that US-guided biopsy should be recommended for large mass lesions, such as those with LPCAL ≥40 mm. In addition, for thoracic lesions with LPCAL <40 mm, the success rate of US-guided vs. CT-guided biopsy was not different, suggesting that US-guided biopsy might be useful in all cases and that the biopsy needle can reach the targets effectively.
Importantly, we observed no severe complications in either procedure. The complication rate for post-procedural pneumothorax and hemorrhage requiring treatment was significantly lower in the US-guided group. US guidance can avoid normal lung through visualization of the lesion with respiratory movement, as well as vessels through color Doppler imaging. Heerink et al. reported that the overall complication rate in CT-guided biopsy was 38.8%. The major complication rate was 5.7%, consisting of pneumothorax requiring intervention, hemothorax, air embolism, needle tract seeding, and death (22). Lee et al. also reported that US-guided biopsy was safer than CT-guided biopsy for peripheral lung lesions larger than 10 mm (23). Therefore, US-guided biopsy might be a safer technique than CT-guided biopsy.
According to these findings, US-guided percutaneous needle biopsy for thoracic lesions may have some advantages with respect to efficacy and safety, compared with the CT-guided procedure. These include: (I) avoidance of radiation exposure; (II) real-time bedside use; (III) repeatability; and (VI) avoidance of vessels with color Doppler imaging. In particular, US-guided percutaneous biopsy was more feasible in cases with bulky lesions (LPCAL ≥40 mm) and equally feasible in cases with non-bulky lesions (LPCAL <40 mm). The presence of a small lesion that cannot easily be detected with US may lead to decreased diagnostic yield or success rates in US-guided biopsy. Therefore, for small lesions, it remains unclear whether US- or CT-guided biopsy is preferable.
The current study had some limitations. The sample numbers were small, and the samples were retrospectively collected. The needle gauge in US-guided biopsy (18 G) was larger than in CT-guided biopsy (19 G), while some reports showed no differences in diagnostic yield according to needle size (24,25). In addition, procedures were selected by each respiratory physician, and patients were not randomized to CT- or US-guided groups, which may result in selection bias. However, new findings by respiratory physicians using these procedures in a clinical setting will be useful, as most of the available data comparing US and CT have previously been derived from procedures performed by radiologists.
Conclusions
In summary, our study showed the feasibility and efficacy of US-guided biopsy for thoracic lesions adjacent to the chest wall. This technique yielded a larger number of samples and a smaller number of post-procedural complications. In patients with LPCAL ≥40 mm, the success rate with US-guided biopsy was higher than that with CT-guided biopsy. Further investigation is needed to confirm which procedure is better in an individual case.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethical Committee of Osaka City University (#1700 and #3433).
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Hori M, Matsuda T, Shibata A, et al. Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2015;45:884-91. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). None small-cell Lung Cancer. Version 3 2017; Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643-52. [Crossref] [PubMed]
- Chavez C, Sasada S, Izumo T, et al. Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: a retrospective comparison between central and peripheral locations. J Thorac Dis 2015;7:596-602. [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-e165S.
- Tian P, Wang Y, Li L, et al. CT-guided transthoracic core needle biopsy for small pulmonary lesions: diagnostic performance and adequacy for molecular testing J Thorac Dis 2017;9:333-43. [Crossref] [PubMed]
- Gupta S, Wallace MJ, Cardella JF, et al. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol 2010;21:969-75. [Crossref] [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6:S99-107. [PubMed]
- Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol 2011;196:W678-82. [PubMed]
- Jarmakani M, Duguay S, Rust K, et al. Ultrasound Versus Computed Tomographic Guidance for Percutaneous Biopsy of Chest Lesions. J Ultrasound Med 2016;35:1865-72. [Crossref] [PubMed]
- Meena N, Bartter T. Ultrasound-guided Percutaneous Needle Aspiration by Pulmonologists: A Study of Factors With Impact on Procedural Yield and Complications. J Bronchology Interv Pulmonol 2015;22:204-8. [PubMed]
- Jeon KN, Bae K, Park MJ, et al. US-guided transthoracic biopsy of peripheral lung lesions: pleural contact length influences diagnostic yield. Acta Radiol 2014;55:295-301. [Crossref] [PubMed]
- Kimura T, Naka N, Minato Y, et al. Oblique approach of computed tomography guided needle biopsy using multiplanar reconstruction image by multidetector-row CT in lung cancer. Eur J Radiol 2004;52:206-11. [Crossref] [PubMed]
- Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology 2013;266:930-5. [Crossref] [PubMed]
- Diacon AH, Schuurmans MM, Theron J, et al. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration 2004;71:519-22. [Crossref] [PubMed]
- Liao WY, Chen MZ, Chang YL, et al. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm in diameter. Radiology 2000;217:685-91. [Crossref] [PubMed]
- Sheth S, Hamper UM, Stanley DB, et al. US guidance for thoracic biopsy: a valuable alternative to CT. Radiology 1999;210:721-6. [Crossref] [PubMed]
- Montaudon M, Latrabe V, Pariente A, et al. Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol 2004;14:1234-40. [Crossref] [PubMed]
- Ohno Y, Hatabu H, Takenaka D, et al. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 2003;180:1665-9. [Crossref] [PubMed]
- Pan JF, Yang PC, Chang DB, et al. Needle aspiration biopsy of malignant lung masses with necrotic centers. Improved sensitivity with ultrasonic guidance. Chest 1993;103:1452-6. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta- analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Lee MH, Lubner MG, Hinshaw JL, et al. Ultrasound Guidance Versus CT Guidance for Peripheral Lung Biopsy: Performance According to Lesion Size and Pleural Contact. AJR Am J Roentgenol 2018;210:W110-8. [Crossref] [PubMed]
- Dooms C, Vander Borght S, Yserbyt J, et al. A Randomized Clinical Trial of Flex 19G Needles versus 22G Needles for Endobronchial Ultrasonography in Suspected Lung Cancer. Respiration 2018;96:275-82. [Crossref] [PubMed]
- Huang ML, Hess K, Candelaria RP, et al. Comparison of the accuracy of US-guided biopsy of breast masses performed with 14-gauge, 16-gauge and 18-gauge automated cutting needle biopsy devices, and review of the literature. Eur Radiol 2017;27:2928-33. [Crossref] [PubMed]


