Preoperative stratification for postoperative delirium: obstructive sleep apnea is a predictor, the STOP-BANG is not?
In the October 2018 issue of the Annals of Thoracic Surgery, Wang et al. published a study intended to test the hypothesis that STOP-BANG can contribute to predict the risk for postoperative delirium (POD) (1). In this paper, the authors report data from an a priori planned sub-analysis of the “PrEventing POstoperative Delirium” (PEPOD) trial: a randomized, double blind, placebo-controlled, single-center clinical trial on the effects of low-dose haloperidol in patients that present POD after major thoracic surgery (protocol NCT02213900). Preoperative obstructive sleep apnea (OSA) was assessed with the STOP-BANG questionnaire administered to all eligible patients that were subsequently categorized into 2 groups: “low risk” (STOP-BANG <3) and “intermediate-to-high risk” (STOP-BANG ≥3). Recorded end points were: incidence and duration of POD (assessed with the confusion assessment method for the ICU: CAM-ICU); occurrence of postoperative coma (assessed through the Richmond Agitation Sedation Scale: RASS); length of ICU and hospital stay. The CAM-ICU is a tool designed and tested to diagnose POD in ICU patients that is considered positive when the following criteria are matched: (I) acute change or fluctuating course of mental status and (II) inattention associated with either (III) altered level of consciousness or (IV) disorganized thinking. The RASS is a 10 stairs scale designed for the evaluation of patient’s arousal—a tool to assess the level of consciousness—that provides values in the range of −5 (unarousable) to +4 (combative), passing for 0 (alert and calm). In the study by Wang et al., patients with RASS scores ≤−4 were considered “comatose” and CAM-ICU evaluation was not accomplished. Patients with RASS ≥−3, were considered eligible for POD assessment and CAM-ICU was evaluated. A total of 128 patients were enrolled and screened for OSA: 31 patients resulted to be at “low risk” and 97 were at “intermediate-to-high”. Results showed no difference between the 2 groups in POD incidence, duration of delirium, length of ICU and hospital stay and days on mechanical ventilation. The cumulative incidence and duration of POD + coma resulted to be higher in patients at “intermediate-to-high” risk for OSA as assessed by the STOP-BANG questionnaire. The authors concluded that a higher preoperative risk for OSA is associated with a 3-fold higher risk for “POD and coma”. Of note, only 20 papers can be retrieved by searching “postoperative delirium AND obstructive sleep apnea” in the PubMed, hence the importance of the preliminary data reported by Wang and coll.
POD is an “acute and fluctuating alteration of mental state of reduced awareness and disturbance of attention” that can occur immediately after the emergence from anesthesia and up to 5 days after surgery (2). Development of POD is associated with worse postoperative outcome and more severe chronic cognitive impairment, longer hospital stays and institutionalization, higher morbidity and mortality (3,4). The incidence of POD is higher in elderly patients and this, associated with the increasing proportion of surgical indications in this subset of patients, makes the clinical relevance of POD a significant challenge in modern anesthesiology and perioperative medicine (2). Occurrence of POD is related to unmodifiable and modifiable risk factors that are for the largest part specific and selective and include: age, cognitive status, chronic use of psychotropic drugs and substances, electrolytes and fluid status abnormalities, history of brain damage or chronic neurodegenerative diseases, etc. (Table 1) (2,3,5-7).

Full table
Among the others, there are evidence that suggest the relevance of preoperative OSA as predictive risk factor for POD, as reported in two studies that enrolled a total of 198 patients (8-11). In the study by Flink et al., data from 106 elderly patients (≥65 years old) undergoing elective knee replacement surgery demonstrated that the incidence of POD was 53% in OSA patients and 20% in those not having OSA among the preoperative risk factors (9). In the study by Roggenbach et al., in 92 patients undergoing cardiac surgery, the risk for POD was 6-fold in those that had preoperative OSA (10). The OSA is a sleep breathing disturbance characterized by multiple episodes of partial or complete upper airway obstruction that cause oxygen desaturation and arousals from sleep, daytime tiredness due to unrefreshing sleep, with a high prevalence in general population (affecting 24% of men and 9% of women), most of them not diagnosed (up to 90%) (12). A diagnosis of OSA is associated with a higher morbidity (coronary artery diseases, hypertension, congestive heart failure, cerebrovascular accidents, gastroesophageal reflux disease), impairment of quality of life, shorter life expectancy and postoperative complications (including: POD, pulmonary complications, cardiac complications, longer hospital length of stay, increased transfer to the intensive care unit, etc.) (12,13). The STOP-BANG questionnaire (sensitivity 84–100% and specificity of 37–56%) is a “concise and easy-to-use screening tool for OSA” (14-16); it is based on the evaluation of both anamnestic and objective findings in physical examination, and it includes 8 items (snoring, daily tiredness or fatigue, observed sleep apnea, high blood pressure, BMI >35 kg/m2, age >50 years, neck >43 cm for men and >41 cm for women, male gender); the presence of 0–2 positive items is associated with a “low risk” for OSA, while 3–4 positive items leads to “intermediate risk” and 5–8 positive items categorize for “high risk” for OSA. Should preoperative OSA can be considered a “modifiable” preoperative risk factor depends on the underlying pathophysiological mechanism in the individual patient. Although, the use of preoperative continuous positive airway pressure (CPAP) therapy have been demonstrated to reduce most of the postoperative OSA-related complications (cardiovascular, pulmonary, etc.) and to shorten the hospital length of stay: it is not clear yet if preoperative CPAP could have a benefit in terms of reduced POD incidence and research in this field is ongoing (12,17,18). In this research field, the “Prevention Of Delirium in Elderly with obstructive Sleep Apnea” (PODESA) study (registration number NCT02954224) by Wong and colleagues is an ongoing multi-center RCT designed to evaluate the impact of auto-titrating APAP in patients scheduled for elective hip or knee replacement surgery with OSA in POD incidence. Of note, preoperative assessment of OSA will be achieved with the STOP-BANG questionnaire: the group with an intermediate and high risk for OSA (STOP-BANG ≥3) will be assessed for OSA with a sleep study and, if positive, will be randomized for APAP treatment or no treatment (17). The study from Strutz and colleagues is an ongoing observational large retrospective study designed to investigate OSA as a risk factor for POD and acute post-surgical pain severity (18). Data from three large RCT will be used for this study, providing an estimate of a total of 1,500 patients: the “Systematic Assessment and Targeted Improvement of Services Following Yearlong Surgical Outcomes Surveys” (SATISFY-SOS) study (registration number NCT02032030), the “Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes” (ENGAGES) study (registration number NCT02241655) and the “Prevention of Delirium and Complications Associated with Surgical Treatments” (PODCAST) study (registration number NCT01690988). Of interest, expected data from this study are not only that patients with a high risk of OSA are at a higher risk for POD but also that they will experience greater postoperative pain severity.
Because of POD clinical relevance, it is important to appropriately accomplish an accurate preoperative risk stratification through the clinical chart evaluation and patient’s interview (2,5,19,20). Accuracy of available preoperative risk stratification tools ranges between 0.52 and 0.94, but none of these include OSA among the recorded variables (20). The possible role of clinical predictors associated with OSA in the preoperative risk stratification for POD is potentially of great clinical relevance and the finding reported by Wang e al., that a higher preoperative OSA risk assessed by the STOP-BANG questionnaire doesn’t relate with actual incidence of POD, might be misleading. We consider that the failure in detecting a clear predictive role of the STOP-BANG for POD, as reported in this study, it is possible due to methodological bias: the study lacks of a specific sample size calculation for the outcomes investigated and could have generated an underpowered statistical planning. Another possible limitation of the study from Wang et al. could lay in the distinct pathophysiology of coma and POD. Finally, even if the CAM-ICU is a validated bedside assessment tool, usable by non-psychiatrists for the detection of POD in ICU, it has a relatively low sensitivity, especially in non-expert hands and the gold standard for the diagnosis is the Diagnostic and Statistical Manual of Mental Disorders or the International Statistical Classification of Diseases and Related Health Problems, performed by experts in the field (2).
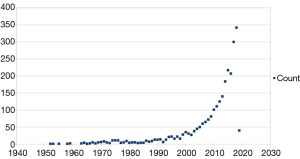
Research in POD boosted in the last years and now, more than 2,600 papers can be retrieved through PubMed using “postoperative delirium” as keyword. The timeline of these publications demonstrates that until 1999 about 20 papers/year were published, thereafter this number progressively increased and reached 300 papers/year in the last 2 years: 300 in 2017 and 344 in 2018 (Figure 1). The growing number of publications in this research field are giving a striking impulse to the progress in understanding the underneath pathophysiology, diagnosis and treatment of POD. Pre-clinical and clinical research projects are addressing various aspect and projects dedicated to the assessment of specific risk factors, prevention and optimal perioperative management, are now undergoing (21-25). The “Postoperative Delirium and Post anesthesia Cognitive Recovery” (PINOCCHIO) Study is an ongoing multi-center randomized clinical trial (RCT), with registration number NCT00507195, designed to detect POD incidence and its link to postoperative cognitive recovery in patients undergoing non-cardiac-non-brain surgical procedures under general anesthesia with different anesthetics (desflurane, sevoflurane and propofol) (21). The “Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep” (MINDDS) trial is an ongoing multi-center RCT, with registration number NCT02856594, designed to evaluate the POD pre-emptive effect of dexmedetomidine-induced sleep in patients undergoing to cardiac surgery with cardiopulmonary bypass (22). The “patient safety, cost-effectiveness, and quality of life: reduction of delirium risk and postoperative cognitive dysfunction after elective procedures in older adults” (PAWEL) study from Sánchez and colleagues is a German 5 medical centers ongoing trial (German Clinical Trials Register number DRKS00013311) that will investigate cross-sectoral and multimodal intervention approach—and its cost/effectiveness ratio—for preventing POD in >70 years old patients undergoing elective surgery (23). The clinical prospective observational study from Momeni et al. was designed to test the hypothesis that a deep intraoperative electroencephalogram (EEG) suppression is related to a higher incidence of POD and a reduced regional cerebral O2 saturation (rScO2) associates with increased risk for postoperative cognitive decline (POCD), that is possibly related to POD; the results by Momeni et al., suggest that intraoperative EEG and of rScO2 monitoring, can effectively contribute to prevent and to diagnose in a timely manner the occurrence of POD and of POCD (24). The “Strategy to Reduce the Incidence of Postoperative Delirium in Elderly Patients” (STRIDE) trial (registration number NCT00590707) from Sieber et al. is an RCT designed to evaluate the effect of depth of sedation in older patients undergoing hip fracture repair on POD, published in the August 2018 issue of JAMA Surgery (25). Results provided from the study indicate that there is no difference in the intraoperative management, in terms of “heavier” or “lighter” level of sedation, in POD incidence; interestingly, in the subgroup analysis, the group with lower comorbidity state exposed to a “heavier” depth of sedation is associated with a 2-fold increased risk of POD incidence, compared to a “lighter” sedation level. These projects demonstrate how important is to run adequately designed clinical studies especially in the evolving research field of POD.
In conclusion, the study by Wang et al. provides interesting and clinically relevant information on the relationship between POD and OSA. Unfortunately, several limitations prevented these Authors to reach the level of evidence that is necessary to formally implement preoperative evaluation of the STOP-BANG questionnaire into the standard of care for POD risk stratification. Future studies are needed to clarify the relevance of preoperative OSA and the accuracy of STOP-BANG questionnaire when used for POD preoperative risk stratification.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wang S, Sigua NL, Manchanda S, et al. Preoperative STOP-BANG scores and postoperative delirium and coma in thoracic surgery patients. Ann Thorac Surg 2018;106:966-72. [Crossref] [PubMed]
- Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017;34:192-214. [Crossref] [PubMed]
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg 2015;220:136-48.e1. [Crossref] [PubMed]
- Bilotta F, Doronzio A, Stazi E, et al. Postoperative cognitive dysfunction: toward the Alzheimer's disease pathomechanism hypothesis. J Alzheimers Dis 2010;22 Suppl 3:81-9. [Crossref] [PubMed]
- Bilotta F, Lauretta MP, Borozdina A, et al. Postoperative delirium: risk factors, diagnosis and perioperative care. Minerva Anestesiol 2013;79:1066-76. [PubMed]
- Hermanides J, Qeva E, Preckel B, et al. Perioperative hyperglycemia and neurocognitive outcome after surgery: a systematic review. Minerva Anestesiol 2018;84:1178-88. [Crossref] [PubMed]
- Raats JW, van Eijsden WA, Crolla RM, et al. Risk Factors and Outcomes for Postoperative Delirium after Major Surgery in Elderly Patients. PLoS One 2015;10:e0136071. [Crossref] [PubMed]
- Gaddam S, Gunukula SK, Mador MJ. Post-operative outcomes in adult obstructive sleep apnea patients undergoing non-upper airway surgery: a systematic review and meta-analysis. Sleep Breath 2014;18:615-33. [Crossref] [PubMed]
- Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012;116:788-96. [Crossref] [PubMed]
- Roggenbach J, Klamann M, von Haken R, et al. Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: a prospective cohort study. Crit Care 2014;18:477. [Crossref] [PubMed]
- Fadayomi AB, Ibala R, Bilotta F, et al. A Systematic Review and Meta-Analysis Examining the Impact of Sleep Disturbance on Postoperative Delirium. Crit Care Med 2018;46:e1204-12. [Crossref] [PubMed]
- Obstructive sleep apnea. Available online: (accessed in 01/02/2019)http://www.stopbang.ca/index.php
- Vasu TS, Doghramji K, Cavallazzi R, et al. Obstructive sleep apnea syndrome and postoperative complications: clinical use of the STOP-BANG questionnaire. Arch Otolaryngol Head Neck Surg 2010;136:1020-4. [Crossref] [PubMed]
- Chung F, Yang Y, Brown R, et al. Alternative scoring models of STOP-BANG questionnaire improve specificity to detect undiagnosed obstructive sleep apnea. J Clin Sleep Med 2014;10:951-8. [PubMed]
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008;108:812-21. [Crossref] [PubMed]
- Chung F, Elsaid H. Screening for obstructive sleep apnea before surgery: why is it important? Curr Opin Anaesthesiol 2009;22:405-11. [Crossref] [PubMed]
- Wong J, Lam D, Choi S, et al. The prevention of delirium in elderly with obstructive sleep apnea (PODESA) study: protocol for a multi-centre prospective randomized, controlled trial. BMC Anesthesiol 2018;18:1. [Crossref] [PubMed]
- Strutz P, Tzeng W, Arrington B, et al. Obstructive sleep apnea as an independent predictor of postoperative delirium and pain: protocol for an observational study of a surgical cohort. Version 2. F1000Res 2018;7:328. [Crossref] [PubMed]
- Borozdina A, Qeva E, Cinicola M, et al. Perioperative cognitive evaluation. Curr Opin Anaesthesiol 2018;31:756-61. [PubMed]
- Lindroth H, Bratzke L, Purvis S, et al. Systematic review of prediction models for delirium in the older adult inpatient. BMJ Open 2018;8:e019223. [Crossref] [PubMed]
- Bilotta F, Doronzio A, Stazi E, et al. Early postoperative cognitive dysfunction and postoperative delirium after anaesthesia with various hypnotics: study protocol for a randomised controlled trial--the PINOCCHIO trial. Trials 2011;12:170. [Crossref] [PubMed]
- Shelton KT, Qu J, Bilotta F, et al. Minimizing ICU Neurological Dysfunction with Dexmedetomidine-induced Sleep (MINDDS): protocol for a randomised, double-blind, parallel-arm, placebo-controlled trial. BMJ Open 2018;8:e020316. [PubMed]
- Sánchez A, Thomas C, Deeken F, et al. Patient safety, cost-effectiveness, and quality of life: reduction of delirium risk and postoperative cognitive dysfunction after elective procedures in older adults-study protocol for a stepped-wedge cluster randomized trial (PAWEL Study). Trials 2019;20:71. [Crossref] [PubMed]
- Momeni M, Meyer S, Docquier MA, et al. Predicting postoperative delirium and postoperative cognitive decline with combined intraoperative electroencephalogram monitoring and cerebral near-infrared spectroscopy in patients undergoing cardiac interventions. J Clin Monit Comput 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Sieber FE, Neufeld KJ, Gottschalk A, et al. Effect of Depth of Sedation in Older Patients Undergoing Hip Fracture Repair on Postoperative Delirium: The STRIDE Randomized Clinical Trial. JAMA Surg 2018;153:987-95. [Crossref] [PubMed]