
Minimized extracorporeal circulation in non-coronary surgery
Introduction
Since the first successful human application of a heart lung machine in 1953 (1) cardiac surgical operations emerged from life-threatening, unpredictable and potentially hazardous methods to well standardized and safe procedures with more or less predictable outcome. The use of cardiopulmonary bypass (CPB) became standard for all operations performed on the arrested open heart. Despite the unopposed advancements of CPB for modern heart surgery it soon became obvious that the application of extracorporeal technology caused adverse effects which negatively influenced the patients’ outcome (2-5). Pathology of CPB was defined by inflammation, activation of the clotting system, haemodilution and formation of micro emboli. These side effects were deemed to result from extensive blood contact with both foreign surfaces and air (open or conventional CPB, CECC). As a consequence, several minimized extracorporeal circulation (MECC) systems were introduced in the late 1990s and early 2000s with the aim to reduce CPB-related morbidity (6-9). Initially thought to be as simple as possible, the first MECC-Systems were reduced to the absolute minimum number of components, the pump and the oxygenator, connected to the patient in a closed loop. This consequently led to a massive reduction in foreign-surface contact and completely abandoned blood-air contact. Thus, the original MECC system was more or less an extracorporeal life support system with the ability to administer cardioplegia (7).
Since then, advancements in perfusion technology along with results derived from perfusion science, especially from studies performed on ‘minimized’ or ‘optimized’ CPB systems, the concept of minimally invasive extracorporeal circulation (MiECC) was established (10). It is important to note that MiECC refers to a combined surgical, anesthesiological and perfusion strategy aiming in the scientifically proven best biocompatibility to the patient. It is not limited to the CPB circuit alone (11). Throughout this article the term ‘MiECC’ will be used synonymously for all kind of minimized CPB systems described in the studies.
In the early days the lack of a venting option restricted the use of the so-called type I MiECC system to coronary surgery only. Moreover, due to the possibility of air entrapment into the venous line and the potential of air embolism, safety concerns prohibited its widespread use in general heart surgery. The integration of passive or active air removal systems (type II) and the possibility of blood pooling without interrupting the principle of the closed-loop perfusion (type III) further enhanced safety and made the MiECC system now suitable for valvular and more complex surgery (12). Modular or hybrid systems (type IV) integrate both a closed-loop and a second open circuit, the latter being used only occasionally in special situations when the features of an open CPB (venous reservoir and other components) are needed. Type IV systems might be useful in very complex cases when high bold loss is anticipated (re-do’s, aortic dissection, endocarditis). Anastasiadis et al. provided an excellent illustrated classification of the MiECC types in their 2016 position paper (11).
Various studies have been conducted to evaluate whether the theoretical advantages of MiECC systems over CECC (less foreign surface contact, no blood-air contact, less hemodilution, less mechanical blood trauma) can be translated in superior clinical outcome. It soon became evident that MiECC, when used in coronary surgery, was able to reduce inflammatory response markers (6), blood loss, and transfusion requirement (9,13-15) as well as length of intensive care unit (ICU) and hospital stay (7,16). In a large-scale meta-analysis on 22.778 coronary artery bypass grafting (CABG) patients. Kowalewski et al. could show that MiECC was associated with a significant reduction of all-cause mortality and stroke and offered a significant protection against postoperative atrial fibrillation and renal dysfunction when compared to CECC (17). These clear and repeatedly proven clinical advantages were interpreted as a result of reduced priming volume and the absence of blood-air interfaces in closed circuits.
However, in patients undergoing non-coronary heart surgery, a vent suction is needed and thus the advantage of the MiECC system could disappear. There are two main drawbacks of minimized circuits when applied in valvular or more complex cardiac surgery, namely: air entrapment from the operating field when the heart chambers are opened, and loss of blood volume due to cardiotomy suction with consecutive low pump flow. It is, therefore, of utmost importance in modern MiECC systems to provide means of air and volume handling without interrupting the principle of closed-loop perfusion.
In the following, the limited scientific evidence of MiECC used in other than coronary surgery is summarized and the first clinical experiences using the concept of modular MiECC are highlighted.
MiECC in valvular and complex heart surgery (selected studies)
Remadi and co-workers were the first to publish the results of a randomized study on 100 patients receiving aortic valve replacement (AVR) using a type II MiECC circuit or a standard CPB (18). Although they could not find differences in hard clinical endpoints (mortality, extubation time, ICU and hospital stay), patients operated on with the MiECC system experienced significantly less release of cardiac troponin and C-reactive protein. Mean arterial pressure was slightly higher during perfusion with the MiECC system with less consumption of inotropes. Haemodilution was attenuated by the MiECC system. However, perioperative blood loss and red blood cell transfusion rates in the two groups were similar.
Focusing on postoperative inflammation Bical et al. randomized 40 patients who underwent isolated AVR (19). They could show only slight differences in pro-inflammatory cytokine release with TNF-α and neutrophil elastase being the only markers which were significantly elevated during reperfusion in open CPB as compared to a type II MiECC system. Patients received neither homologous nor red blood cell products in both groups. Although MiECC was associated with a somewhat lesser inflammation response, there were no immediate clinical benefits seen in the study.
The same type II MiECC system was used in a study performed by Castiglioni and colleagues, who randomized 40 consecutive patients undergoing surgical AVR (20). Patients in the MiECC group (n=17) showed reduced blood loss, reduced transfusion requirements, and higher haematocrit levels compared with patients in the standard CPB group. Interestingly, MiECC patients experienced a longer hospital stay, which was probably due to a disabling stroke occurring in the MiECC group. The researchers introduced pulmonary artery venting instead of pulmonary vein venting as a means of unloading the left ventricle without interrupting the closed circuit. However, they admitted, that the sample was not large enough to demonstrate the safety of the procedure. Therefore, 2 years later the same group published the data of another prospective-randomized study now on 120 patients (21). Again, they could find significant advantages of MiECC in terms of blood loss, need for blood transfusions, platelet consumption, and myocardial damage. In-hospital mortality, major neurological events, ventilation time, ICU stay, and hospital stay did not differ between the groups.
With the purpose to evaluate the safety and clinical outcome of AVR using either standard or minimized CPB Colli et al. (22) performed a cohort study on 128 patients of whom 53 patients were operated on using a type III MiECC system. The decision to use MiECC or standard CPB was made on the surgeon’s preference. The MECC system contained a vacuum bag to collect the blood returned from the vent placed in the right superior pulmonary vein. Because of the ‘partial air-blood-contact’ the authors considered their system ‘semi-closed’. Patients in both groups showed similar postoperative chest tube drainage and transfusion requirements. Perioperative mortality, renal injury, atrial fibrillation, stroke, ICU and hospital stay were comparable in both groups.
Yilmaz and co-workers published a series of 50 patients who underwent combined minimal access AVR (upper partial sternotomy) with a type II MiECC system (23). They used the groin vessels for CPB access, combined arterial and venous RAPing and a pulmonary artery vent, which was re-routed into the venous line of the MiECC system. An aortic root vent was connected to a cell saver. With this technique they could achieve a hundred percent clinical success; no conversions to full sternotomy nor to open CPB were recorded. Perioperatively only one blood transfusion was required.
Using a self-designed type III MiECC system which was built of components from 4 different manufacturers. Ariyaratnam et al. operated on 187 AVR patients (including 67 patients receiving AVR + CABG) and 7 mitral valve repair (MVR) patients. They reported the outcomes of these patients within a retrospective propensity-matched analysis in comparison to patients who were operated on using conventional CPB (24). Patients in the MiECC group had significantly longer bypass and cross-clamp times and higher rates of postoperative atrial fibrillation. On the other hand, the mean transfusion rate of blood cells was significantly reduced with MiECC. The overall 5-year survival was higher in the MiECC group.
In order to demonstrate the feasibility of MiECC in more complex surgery than AVR. Momin and colleagues published the results of 49 patients undergoing major aortic surgery using a type III MiECC system based on a modified Sorin ECCO mini-CPB (25). The ‘Hammersmith mini-CPB system’ was built with a set of standard components but customized according to the needs of the patients. A venous air removal device facilitated the management of gross and microscopic air bubbles. Versatility in complex procedures was enhanced by an optional (parallel) soft shell reservoir. Clinical outcome data were compared to those of 328 consecutive patients having similar surgery with conventional CPB at the same time. There were no differences in perioperative mortality, blood consumption, renal or neurological complications between the groups.
Anastasiadis et al. published a series of three patients undergoing major aortic surgery and left ventricular assist device (LVAD) implantation under MiECC support. They could show the feasibility and safety of minimized perfusion even in very complex surgical procedures (26).
Recently, Gygax et al. (27) came up with a prospective randomized study on 50 patients undergoing AVR either with a type II MiECC system (n=24) or a conventional CPB (n=26). The study aimed to compare both groups with respect to markers of complement activation (sC5b-9), inflammation (IL-6, TNF-α, sCD40-ligand), and coagulation activation (D-dimer, TAT). All the parameters tested did not differ between groups during the time course. Likewise, the incidence of clinical complications (atrial fibrillation, stroke) was similar.
The same group published their data on cerebral microembolization during AVR using the two types of perfusion (28). A total of 48 patients were randomized in a 1:1 ratio to MiECC (type II) or conventional CPB and high-intensity transient signals (HITS) count was measured during perfusion using transcranial Doppler. The generation of gaseous microemboli was significantly enhanced when MiECC was used. The overall HITS rate was increased by a factor of 1,75 in the MiECC group. Post hoc analysis revealed that in particular oxygenator types without integrated arterial filter tended to be associated with higher HITS rates.
Modular MiECC (type IV)
The first to describe the principle of modular MiECC were El-Essawi and colleagues (29) who published the results of a prospective, randomized, multicentre clinical trial (291 patients) using the ROCsafeRXTM system (Terumo Cardiovascular Systems, Ann Arbor, MI, USA). This minimized closed-loop circuit incorporated not only the two essential components centrifugal pump and low-resistance oxygenator but also a 40-µm arterial line filter, a venous de-airing unit, a bubble trap and an electronic venous line occluder. The modular concept comprised a pre-connected hard-shell reservoir supplemented by a pericardiotomy suction, that could be integrated by quick connectors in case of a massive air leak or major bleeding. Venting was accomplished via the pulmonary vein directly to the venous line or into a flexible reservoir. One hundred forty-six patients were randomized to be operated on with the modular MiECC system including AVR (n=25) and AVR + CABG (n=14). One hundred forty-five patients were operated on using conventional CPB. The distribution of patients receiving AVR or AVR + CABG was balanced between the groups. Although all procedures were done with a cell saving device, only 31.5% of patients in the MiECC group had to be re-transfused with washed red cells. Postoperative blood loss at 12 hours was comparable between the groups (MiECC 515±359 vs. CPB 557±421 mL, n.s.). The need for blood transfusion was significantly lower in the MiECC group (329±599 vs. 789±1,638 mL; P<0.001). MiECC patients had fewer episodes of atrial fibrillation (7.1% vs. 19,5%; P<0.001). Other clinical outcome-parameters (in-hospital mortality, stroke, myocardial infarction, ICU and hospital stay) did not differ between the groups.
The same group recently published the results of a matched-pair analysis on 104 octogenarians receiving AVR either with a modular type IV MiECC system or standard CPB (30). The 30-day mortality tended to be reduced in the MiECC group (2% vs. 10%; P=0.2). Likewise, hemodynamic performance and ICU stay were in favor of MiECC without being statistically significant. However, 90-day mortality was clearly better in the MiECC group (2% vs. 16%; P=0.02).
Anastasiadis et al. (31) designed a modular type IV MiECC circuit containing an accessory circuit for immediate transition to an open system offering enhanced safety features (AHEPA circuit). Based on Medtronic Affinity components (centrifugal pump and membrane oxygenator with integrated arterial filter) the system contained a soft bag, two bubble traps, a venous air removal device, and a standby hard-shell reservoir. The AHEPA system was used in a series of 50 consecutive patients, of whom 27 patients received procedures other than elective CABG (AVR, MVR, operations on the tricuspid valve and the ascending aorta). Almost all patients (96%) could be operated on using the minimized closed circuit. Two patients required conversion to open CPB (one re-do, one acute aortic dissection). Overall mortality was 4% (2 patients; not the ones who had to be converted). Comparing the results of modular MiECC to those of a historic cohort of high-risk CABG patients operated on with a standard MiECC system (n=100), the authors stated that the modular configuration did not influence the favourable characteristics attributed to MiECC. In particular, hemodynamic integrity (lower haemodilution, higher perfusion pressure) could be preserved irrespective of the kind of surgical procedure.
An overview of the studies analysed is given in Table 1.
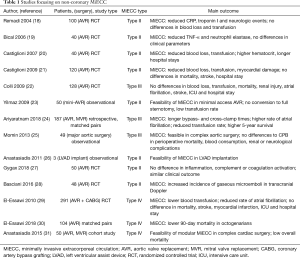
Full table
The Ulm University modular MECC system
The system used at Ulm University comprises a minimized closed-loop main circuit and a second open circuit bridging the main circuit in parallel (Figure 1). The main circuit contains a centrifugal pump (BB Affinity Pump CBBPX-80, Medtronic Inc., Minneapolis, MN, USA), a membrane oxygenator with integrated arterial filter (CB841, Medtronic Inc.), a venous air removal device (CB VARD AAR1000; Medtronic Inc.), and a 2-liter soft bag for volume buffering and RAPing. The vent is connected to the VARD. Myocardial protection is accomplished with warm blood cardioplegia (Calafiore) which is administered with a syringe pump directly into the CP line coming from the oxygenator outlet. The second circuit contains only a hard-shell reservoir connected in parallel to the venous line. In- and outflow is regulated manually by two clamps. Thus, the system can be converted from a closed to an open system within a second. If a cardiotomy suction is used, it can be decided whether the shed blood is recirculated to the system or discarded. All the lines are surface-coated (Carmeda BioActive Surface, Medtronic Inc.). A cell-saver device is regularly added to the circuit. It receives blood/air aspirate from the aortic root vent or the left atrial vent. In case a pulmonary artery vent is used, it is directly connected to the VARD. The whole system is run on a Stöckert CP5 console (LivaNova Inc., Milano, IT, USA).
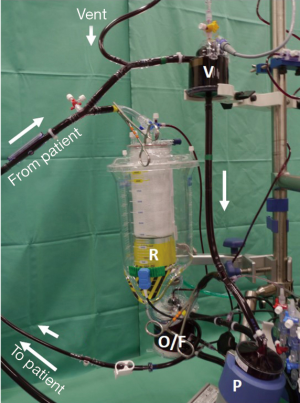
We used this modular MiECC configuration in a series of 48 consecutive patients receiving a variety of non-coronary procedures including atrial fibrillation ablation (Table 2). Since mitral valve surgery in our institution is performed in minimally invasive, videoscopically enhanced technique, no isolated mitral valve procedures are contained in this series. Technical success was 100%. In most cases (44/48 patients) only the main circuit was utilized; in 4 patients we needed to open the circuit for the use of the reservoir. The cell saver was in use from the beginning in all of the procedures. When the amount of blood in the cell saver reservoir exceeded 500 mL, the blood was washed and recirculated. This was the case in nearly half of the patients (23/48 patients). In the remaining half (25/48 patients) the shed blood was discarded. The blood collected in the hard-shell reservoir (4/48 patients) was directly recirculated. Although perioperative blood loss was low (450±330 mL) the majority of patients (39/48 patients; 81%) received blood transfusions during the postoperative course. This might be the result of a rather liberal transfusion policy in our institution without strict lower limits for haematocrit. No major clinical adverse events (death, stroke, myocardial infarction) were observed. Three patients (6%) showed temporary signs of productive delirium; one patient had to be re-explored for a surgical bleeding. Fourteen patients (29%) developed new atrial fibrillation; all but one of them could be converted to SR during the hospital stay*.
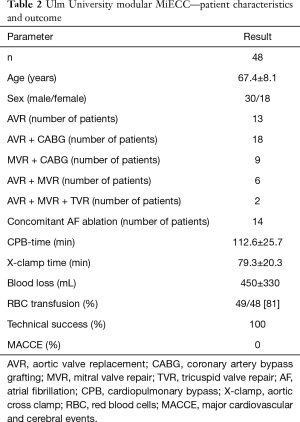
Full table
Comments
Summarizing the studies that used a type II or III minimized CPB circuit on patients undergoing aortic valve surgery the typical clinical benefits found in CABG surgery were not demonstrable in the same way. This may be due to the fact that these small mostly single-institutional trials used different technologies and were powered for feasibility only or for demonstration of subclinical effects (e.g., inflammation, coagulopathy, haemodilution) that potentially could have an impact on postoperative morbidity and mortality.
A variety of MiECC systems provided by different manufacturers (Maquet - Getinge, Sorin - LivaNova, Medtronic, Terumo) with different configurations were used in the studies. Some researchers designed their own MiECC system combining components from different companies (24,25). In one study three different oxygenators were utilized in the MiECC group (27). The use of the vent was mostly variable. It was placed in the pulmonary vein (7,21) or in the pulmonary artery (19,23); it was connected to a vacuum bag (7,22), alternatively to the cell saver (21), or to a small cardiotomy reservoir (19).
Blood processing was also different between the studies. In some studies, the cell saver was used as the only suction device in the CPB group (7,19,23); in other studies, it was allowed in both groups (19,22,27). Whether or not and to what extent the shed blood was discarded or utilized was sparsely documented. Likewise, the concept of retrograde autologous priming (RAP) was differently handled. Some authors followed the principle of complete arterial and venous RAPing very strictly irrespective of hemodynamic impairment; if necessary hypotension was counteracted with norepinephrine. Others abstained completely from RAPing.
Regarding blood transfusions as a main outcome parameter it is of major importance that no rigid transfusion trigger has been applied in most clinical studies. Thus, it is not surprising that the blood sparing effect of MiECC was not seen in all studies. Unlike in CABG surgery, where all researchers uniformly reported on significant reduction of blood loss by MiECC, in non-coronary surgery a considerable number of trials showed the same blood loss and transfusion need like in conventional CPB (22,23,32). In one study, a significantly higher number of patients in the CPB group had to be transfused even though the perioperative blood loss was the same (29). This possibly reflects the better preservation of cellular blood components by using a minimized circuit with less damaging components and complete surface coating.
A major benefit of all types of MiECC was the reduction of priming volume and the shortening of tubing length. This greatly reduced foreign surface contact and haemodilution. However, haemodilution was not only influenced by the priming solution [priming volumes varied from 200 (23) to 900 mL (22) in the different MiECC configurations] but also by intravenous volume replacement during anaesthesia induction. This circumstance was rarely assessed by the investigators.
Organ protection is a major issue in extracorporeal perfusion. The formation of microemboli can lead to disturbances in microcirculation and consequently to organ dysfunction. Unlike in CABG surgery (33,34), which is performed on the surface of the heart, open heart surgery is associated with a larger cerebral microembolic burden (28). It is assumed that under the circumstances of MiECC (excessive negative line pressures) the embolic load is even higher. In the attempt to fully unload the heart spontaneous formation of microbubbles by cavitation is possible. Another source of emboli in open heart surgery, especially in AVR, may be remaining amounts of air in the apical and septal region of the left ventricular cavity even after meticulous de-airing. It is therefore of utmost importance to add an arterial line filter. Conservative negative line pressure limits and consequent CO2 flooding can add to safety.
Two meta-analyses, with different methodologies, have addressed clinical implications of minimized CPB in valvular heart surgery (35,36). The first meta-analysis was published in 2013 and comprised 24 randomized controlled trials (RCTs) involving a total of 2,770 patients (1,387 allocated to MiECC vs. 1,383 allocated to standard CPB) of whom 721 patients underwent non-coronary surgery (AVR). Overall mortality rate was 0.5% in the MiECC group and 1.7% in the standard CPB group (P=0.02). This significant difference was attributed to CABG procedure only, while no difference in mortality was found in AVR patients. Similarly, myocardial infarction and neurologic events occurred equally frequent in AVR patients irrespective of the perfusion mode. Regarding secondary outcome parameters (inflammation, blood loss and transfusion, renal function, low cardiac output, time on ventilator, ICU and hospital stay) due to its design this meta-analysis did not allow to draw a definite conclusion on the superiority of one of both perfusion modes in AVR patients (35).
The to date latest meta-analysis focusing on miniaturized CPB in valve surgery was published in 2016. Eight RCTs involving 1.011 patients were studied. As a result, the authors stated that application of MiECC in valve surgery significantly reduced ICU and total hospital stay. However, no significant effects on reducing mortality and postoperative morbidity (neurologic events, arrhythmias, inotropic support) were observed. The effects of MiECC on blood loss and transfusion requirements were not addressed in this study (36).
In conclusion, the relevant literature suggests, that MiECC can safely be used for non-coronary surgery. The question whether the use of MiECC in other than coronary surgery influences patients’ outcome (morbidity and mortality) remains unanswered. The ambitious standards that we impose in complex intracardiac procedures must consider measures for volume unloading and air handling as well as the possibility to react on unforeseeable events. This, together with the high demand on safety renders a modular approach indispensable. We believe that the preliminary good results justify carrying out a multicentre prospective randomized trial with the use of the latest MiECC technology in non-coronary surgery.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gibbon JH. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med 1954;37:171-85. [PubMed]
- Cheng DC. The systemic inflammatory response to cardiac surgery. Anesthesiology 2002;97:215-52. [Crossref] [PubMed]
- Westaby S. Organ dysfunction after cardiopulmonary bypass. A systemic inflammatory reaction initiated by the extracorporeal circuit. Intensive Care Med 1987;13:89-95. [Crossref] [PubMed]
- Murphy GJ, Angelini GD. Side effects of cardiopulmonary bypass: what is the reality? J Card Surg 2004;19:481-8. [Crossref] [PubMed]
- Levy JH, Tanaka KA. Inflammatory response to cardio- pulmonary bypass. Ann Thorac Surg 2003;75:S715-20. [Crossref] [PubMed]
- Fromes Y, Gaillard D, Ponzio O, et al. Reduction of the inflammatory response following coronary bypass grafting with total minimal extracorporeal circulation. Eur J Cardiothorac Surg 2002;22:527-33. [Crossref] [PubMed]
- Wiesenack C, Liebold A, Philipp A, et al. Four years’ expe- rience with a miniaturized extracorporeal circulation system and its influence on clinical outcome. Artif Organs 2004;28:1082-8. [Crossref] [PubMed]
- Abdel-Rahman U, Ozaslan F, Risteski PS, et al. Initial experience with a minimized extracorporeal bypass system: is there a clinical benefit? Ann Thorac Surg 2005;80:238-43. [Crossref] [PubMed]
- Remadi JP, Rakotoarivelo Z, Marticho P, et al. Prospective randomized study comparing coronary artery bypass grafting with the new mini-extracorporeal circulation Jostra system or with a standard cardiopulmonary bypass. Am Heart J 2006;151:198. [Crossref] [PubMed]
- Anastasiadis K, Bauer A, Antonitsis P, et al. Minimal invasive Extra-Corporeal Circulation (MiECC): a revolutionary evolution in perfusion. Interact CardioVasc Thorac Surg 2014;19:541-2. [Crossref] [PubMed]
- Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 2016;22:647-62. [Crossref] [PubMed]
- Yilmaz A, Sjatskig J, van Boven WJ, et al. Combined coronary artery bypass grafting and aortic valve replacement with minimal extracorporeal closed circuit circulation versus standard cardiopulmonary bypass. Interact CardioVasc Thorac Surg 2010;11:754-7. [Crossref] [PubMed]
- Asteriou C, Antonitsis P, Argiriadou H, et al. Minimal extracorporeal circulation reduces the incidence of postoperative major adverse events after elective coronary artery bypass grafting in high-risk patients. A single-institutional prospective randomized study. Perfusion 2013;28:350-6. [Crossref] [PubMed]
- Biancari F, Rimpilainen R. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart 2009;95:964-9. [Crossref] [PubMed]
- Harling L, Warren OJ, Martin A, et al. Do miniaturized extracorporeal circuits confer significant clinical benefit without compromising safety? A meta-analysis of randomized controlled trials. ASAIO J 2011;57:141-51. [Crossref] [PubMed]
- Rimpiläinen R, Biancari F, Wistbacka J, et al. Outcome after coronary artery bypass surgery with miniaturized versus conventional cardiopulmonary bypass. Perfusion 2008;23:361-7. [Crossref] [PubMed]
- Kowalewski M, Pawliszak W, Raffa GM, et al. Safety and efficacy of miniaturized extracorporeal circulation when compared with off-pump and conventional coronary artery bypass grafting: evidence synthesis from a comprehensive Bayesian-framework network meta-analysis of 134 randomized controlled trials involving 22 778 patients. Eur J Cardiothorac Surg 2016;49:1428-40. [Crossref] [PubMed]
- Remadi JP, Rakotoarivello Z, Marticho P, et al. Aortic valve replacement with the minimal extracorporeal circulation (Jostra MECC System) versus standard cardiopulmonary bypass: a randomized prospective trial. J Thorac Cardiovasc Surg 2004;128:436-41. [Crossref] [PubMed]
- Bical OM, Fromes Y, Gaillard D, et al. Comparison of the inflammatory response between miniaturized and standard CPB circuits in aortic valve surgery. Eur J Cardiothorac Surg 2006;29:699-702. [Crossref] [PubMed]
- Castiglioni A, Verzini A, Pappalardo F, et al. Minimally invasive closed circuit versus standard extracorporeal circulation for aortic valve replacement. Ann Thorac Surg 2007;83:586-91. [Crossref] [PubMed]
- Castiglioni A, Verzini A, Colangelo N, et al. Comparison of minimally invasive closed circuit versus standard extracorporeal circulation for aortic valve replacement: a randomized study. Interact Cardiovasc Thorac Surg 2009;9:37-41. [Crossref] [PubMed]
- Colli A, Fernandez C, Delgado L, et al. Aortic valve replacement with minimal extracorporeal circulation versus standard cardiopulmonary bypass. Interact Cardiovasc Thorac Surg 2009;9:583-7. [Crossref] [PubMed]
- Yilmaz A, Rehman A, Sonker U, et al. Minimal access aortic valve replacement using a minimal extracorporeal circulatory system. Ann Thorac Surg 2009;87:720-5. [Crossref] [PubMed]
- Ariyaratnam P, Mclean LA, Cale A, et al. Mini-extracorporeal circulation technology, conventional bypass and prime displacement in isolated coronary and aortic valve surgery: a propensity-matched in-hospital and survival analysis. Interactive CardioVascular Thoracic Surgery 2018;27:13-9. [Crossref] [PubMed]
- Momin AU, Sharabiani M, Kidher E, et al. Feasibility and safety of minimized cardiopulmonary bypass in major aortic surgery. Interact CardioVasc Thorac Surg 2013;17:659-63. [Crossref] [PubMed]
- Anastasiadis K, Chalvatzoulis O, Antonitsis P, et al. Use of minimized extracorporeal circulation system in non-coronary and valve cardiac surgical procedures — a case series. Artif Organs 2011;35:960-3. [Crossref] [PubMed]
- Gygax E, Kaeser HU, Stalder M, et al. Type II Minimal-Invasive Extracorporeal Circuit for Aortic Valve Replacement: A Randomized Controlled Trial. Artif Organs 2018;42:620-9. [Crossref] [PubMed]
- Basciani R, Kröninger F, Gygax E, et al. Cerebral Microembolization During Aortic Valve Replacement Using Minimally Invasive or Conventional Extracorporeal Circulation: A Randomized Trial. Artif Organs 2016;40:E280-91. [Crossref] [PubMed]
- El-Essawi A, Hajek T, Skorpil J, et al. A prospective randomised multicentre clinical comparison of a minimised perfusion circuit versus conventional cardiopulmonary bypass. European Journal of Cardio-thoracic Surgery 2010;38:91-7. [Crossref] [PubMed]
- El-Essawi A, Breitenbach I, Haupt B, et al. Aortic valve replacement with or without myocardial revascularization in octogenarians. Can minimally invasive extracorporeal circuits improve the outcome? Perfusion 2019;34:217-24. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Argiriadou H, et al. Modular minimally invasive extracorporeal circulation systems; can they become the standard practice for performing cardiac surgery? Perfusion 2015;30:195-200. [Crossref] [PubMed]
- Kobayashi Y, Mitsuno M, Yamamura M, et al. Evaluation of closed cardiopulmonary bypass circuit for aortic valve replacement. ASAIO J 2010;56:309-12. [PubMed]
- Liebold A, Khosravi A, Westphal B, et al. Effect of closed minimized cardiopulmonary bypass on cerebral tissue oxygenation and microembolization. J Thorac Cardiovasc Surg 2006;131:268-76. [Crossref] [PubMed]
- Perthel M, Kseibi S, Sagebiel F, et al. Comparison of conventional extracorporeal circulation and minimal extracorporeal circulation with respect to microbubbles and microembolic signals. Perfusion 2005;20:329-33. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Haidich AB, et al. Use of minimal extracorporeal circulation improves outcome after heart surgery; a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2013;164:158-69. [Crossref] [PubMed]
- Wang C, Hua K, Yin L, et al. A Meta-Analysis of miniaturized versus conventional extracorporeal circulation in valve surgery. Ann Thorac Surg 2016;102:2099-108. [Crossref] [PubMed]


