Devising the guidelines: the concept of uniportal video-assisted thoracoscopic surgery—incisions and anesthetic management
Introduction
Uniportal video-assisted thoracoscopic surgery (VATS) is an already established minimally invasive technique in the field of thoracic surgery. The feasibility, safety and efficacy of the technique are already well documented. Comparative studies and meta-analyses have shown a clear advantage over open surgery and other minimally invasive techniques in terms of pain, length of stay (LOS), chest drain duration and morbidity. (1). It covers a broad spectrum of indications for both malignant and benign diseases, major and minor thoracic procedures, including pulmonary and mediastinal tumor resections, diaphragm procedures (plication), esophageal surgery, distal airway surgery (bronchial resections, carinal resections), pleural disease (pneumothorax, empyema) and palmar hyperhidrosis (sympathectomy) (2-9). Its swift and wide adoption has resulted into many variations, all of whom are common in the fact they utilize a single incision to enter the chest and conduct the planned procedure. With this article, we attempt to standardize the technique as to the incision and the anesthetic management.
Incision
Pulmonary resections
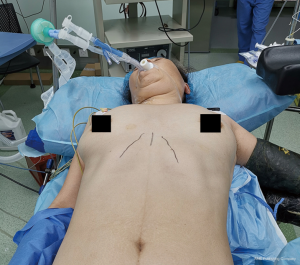
Generally, it is recommended that the incision is sited at the fifth intercostal space (ICS), slightly anteriorly, somewhere between the middle and anterior axillary line (Figure 1). (10). From that level, one can have a direct access to the fissure and convenient angles for the transection of the lobar bronchovascular structures and especially the vein. However, harvesting the lymph node stations 4R and 2R, especially the later, may pose some difficulties when attempted via the fifth ICS, mainly due to the fact that the instruments and the camera may align, which can result to fencing. The 4th ICS, although preferred by some surgeons, can be inconvenient for the stapling of the superior pulmonary vein (right and left), because of the perpendicular axis from the incision to the vessel, which obliterates the angle required to negotiate around it, but can make the 4R and 2R easier to dissect (11). For bronchial sleeve resections, the 4th ICS offers the surgeon an axis almost perpendicular to the axis of the bronchial anastomosis, thus making it ideal for suturing the anastomosis (6). This applies for all the lobes (upper, middle, lower) in both lungs (right, left). Either in the 4th or 5th ICS the incision does not interfere with the mammary gland.
Siting the incision anteriorly may contribute to less postoperative pain, because the more anterior, the wider the ICS tends to be, resulting to less nerve injury. One should keep in mind that siting the incision too anteriorly may limit one’s access to the posterior mediastinal pleura, which is often incised to facilitate the dissections required for lower and left upper lobectomies. Limited access to the posterior mediastinum, also means less effective lymph node dissection of the stations 7, 8 and 9.
The length of the incision can vary from 3–6 cm, depending on the preference and expertise of the surgeon, as well as the size of the tumor and the subcutaneous fat. Incisions smaller than 3 cm may limit the surgeon’s ability to effectively maneuver his instruments. Particularly for large tumors, a single cut near the anterior end of the rib can facilitate the delivery of the specimen without further enlargement of the incision (12). Incisions larger than 6 cm can have as a result the loss of support, especially for the dissecting instrument (hook, energy device, dissector) which is usually anchored at the most inferior part of the incision and/or the camera which is locked at the most posterior part of the incision (10). In order to dismiss any definition issues, it is worthy to note that a resection performed via an incision up to 8 cm is still considered a VATS resection according to the Cancer and Leukemia Group B (CALGB) 39802 trial of the American Society of Clinical Oncology (13).
After incising the skin and the subcutaneous tissue, care should be taken to preserve the integrity of the underlying serratus anterior muscle. This can further reduce the surgically induced trauma. The serratus’s muscle fibers are separated, rather than cut, until the intercostal muscles are visible. The intercostal muscles are incised at the superior rim of the inferior rib, to avoid injuring the overlying neurovascular bundle, running along the inferior rim of the superior rib.
For the resection of the basal segments (e.g., segment 8, 9 or 10) and especially segment 8, a difficult step of the procedure is the division of the intersegmental plane between these segments. Although not routinely used, an incision sited at the sixth ICS at the level between the middle and anterior axillary line may facilitate this procedure, because it is closer to the inferior rim of the lung. However, a careful assessment of the position of the diaphragm should be attempted firstly.
For wedge resections, the incision can be adjusted according to the position of the lesion to be resected. For example, for a lesion located to the apical segment (left or right) the fourth ICS may be more convenient than the fifth. If the lesion is situated posteriorly, again the surgeon may choose to site his incision more laterally.
Mediastinal tumor resections
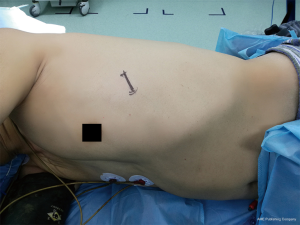
For tumors situated in the anterior mediastinum and for thymectomies, the fourth ICS at the level of the anterior axillary line provides adequate access to the entire compartment, facilitating dissection of the structure from the level above the innominate vein to the cardiophrenic angles (Figure 2) (3,14). However, when dissection above the level of the innominate vein is not required, or when the tumor is situated at the lower part of the anterior mediastinum, the fifth ICS could be more convenient. Masses protruding to one or the other side of the chest should be accessed accordingly. The positioning of the patient on the operating table, is also very significant, as it differs than that required for pulmonary resection. A degree of posterior inclination is essential. The patient is usually secured in a semi-supine position, with the ipsilateral hand abducted and suspended above the head from an L-shaped support, while the surgeon is positioned behind him/her. Generally, preoperative planning with the help of multi-detector computed tomography (MDCT) images is mandatory before siting the incision.
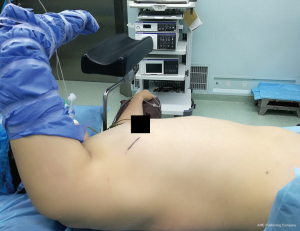
For posterior mediastinal masses, the ICS incised depends on the location and the size of the mass. An incision at the level of the 4th–6th ICS, middle axillary line should provide adequate access. The patient is placed in a semi-prone position and the surgeon stands in front of him/her. If a lateral decubitus position is selected instead, a forward tilt of the operating table (towards the surgeon), usually facilitates access to the target area.
When the tumor is located in the middle mediastinum, an incision at the fourth ICS is recommended, if it is at the level, or above, of the azygos (right side) or aortic arch (left side). Otherwise, the fifth ICS is an equally convenient alternative.
For mediastinal tumors involving the thoracic outlet, the fourth ICS could produce inconvenient acute angles, resulting to alignment of the instruments and fencing. Thus, incising the third ICS between the middle and anterior axillary line, could be more beneficial. The lateral decubitus is usually the position of choice.
Subxiphoid uniportal VATS
Uniportal VATS in considered the least invasive access. However, because the access is achieved via the ICS, it inevitably induces a certain degree of intercostal nerve injury, which can lead to symptoms such as pain and chest wall paresthesia. These symptoms often persist long after the discharge of the patient (15). To tackle this issue an alternative subxiphoid access was attempted, leading to the evolution of a new minimally invasive technique, the subxiphoid uniportal VATS.
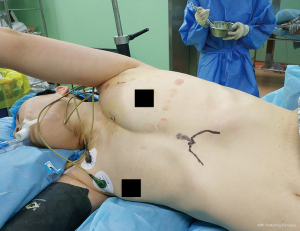
In this technique access to the pleural cavity is achieved via a 3–4 cm vertical incision, just above the xiphoid process and extending up to the end of it (16,17). A transverse incision has also been reported (17). When a transverse incision is utilized the rectus abdominis has to be incised transversely, while for a vertical incision the rectus abdominis is detached from the xiphoid process (16,17). There seems to be no other particular advantage of the vertical versus the transverse incision, apart from maintaining the integrity of the rectus abdominis. The xiphoid process is usually removed. The patient is positioned in the lateral decubitus position with a slight posterior inclination, or in the supine position, with a slight inclination anteriorly for lung resections and in the supine position for anterior mediastinal tumor resections (16-18) (Figures 3,4). Elevation of the sternum from its distal end and from the sternal notch, via specially designed retractors, has been described in order to provide additional space for the dissection of the thymus and mediastinal fat (19).
Anesthetic management
The anesthetic management, regarding ventilation, for uniportal VATS does not differ from that utilized for other minimally invasive thoracic surgery techniques. One-lung ventilation (OLV) is mandatory and is achieved via a double lumen endotracheal tube (DLT), consisting of a tracheal and bronchial component. Before the onset of OLV, the patient should be ventilated with 100% oxygen for 3–5 minutes, in order to de-nitrogenize the lungs and facilitate the effective collapse of the non-dependent lung. (20). Intermittent suction can also accelerate and maintain the collapse. Generally low tidal volumes (6–8 mL/kg) and positive end expiratory pressure (PEEP) of 5 cmH2O are used during ventilation (21).
Despite the fact that uniportal VATS is the least invasive technique regarding the number of incisions and is associated with lower postoperative pain scores compared to other minimally invasive techniques and of course thoracotomy, the thorn of postoperative pain is still a matter that may impair postoperative recovery (22). Post-VATS pain is associated with intercostal nerve injury, which seems to be inherent to any intercostal surgical technique. Consequently, a variety of strategies have been developed to tackle or minimize it. These include: pre-emptive infiltration of the wound, paravertebral block (PVB), intercostal nerve block (ICNB), serratus anterior block (SAB), thoracic epidural anesthesia (TEA). Our knowledge for the efficacy of these techniques in the management of postoperative pain, mainly derive from open surgery (thoracotomy) studies.
Pre-emptive infiltration of the wound
Infiltrating the site of the incision with a local anesthetic agent prior to the incision has been related with significantly less postoperative pain. Sihoe et al. prospectively investigated the effects of 10 mL 0.5% bupivacaine on postoperative pain in 31 patients who underwent bilateral needlescopic VATS sympathectomy. Patients reported significantly less pain in the infiltrated side compared to the control side at 7 days after surgery (23). The same conclusion was reached by Fiorelli et al. who used 2% lidocaine and epinephrine 5 minutes prior to the incision (24).
PVB
PVB in a valuable tool in the treatment of postoperative pain. Again, local anesthetic agents such as bupivacaine and ropivacaine are the most commonly used ones. These agents are infused into the space adjacent to the spine where the intercostal nerves emerge. Because this space is not covered by the intrathoracic fascia the nerves are more susceptible to the effects of these agents. Single shot instillation or continuous effusion via catheters can be used. The paravertebral space can be accessed percutaneously with or without the guidance of ultrasound or under direct vision after VATS.
Wu et al. randomized 171 patients into a patient controlled PVB group and to a patient-controlled analgesia (PCA) group (86 and 85 patients respectively). For the PVB group an epidural catheter was placed to an “extrapleural pocket” created carefully by dissecting the parietal pleura off the chest wall. A 300 mL solution containing 500 mg ropivacaine was inserted into the pump. The pump was set to continuously deliver 10 mg/h of ropivacaine. When the patient was in pain a bolus of 2-mg ropivacaine was infused. The PCA group was offered sufentanil continuously at a rate of 2 µg/h, and a 1.5 µg bolus dosage when in pain. Although the pain scores between the two groups did not differ at 6, 24, 48 and 72 hours postoperatively, the PVB group consumed less dezocine (rescue medication) when in pain than the PCA group. Moreover, severe vomiting and hypotension was significantly lower at the PVB, which also exhibited a significantly higher patient satisfaction score (25). Komatsu et al. administered a continuous effusion of 0.5% bupivacaine hydrochloride or 0.2% ropivacaine hydrochloride into the paravertebral space following a bolus of 15 mL of bupivacaine or ropivacaine respectively at the completion of the chest wall closure in 115 patients. The authors reported that 82% of the patients tolerated the postoperative pain with only NSAIDs p. os, while the rate of pain related postoperative pulmonary complications were zero (26).
ICNB
The block of intercostal nerves with local anesthetic agents is a well-known anesthetic technique in thoracic surgery. It is simple and safe and can be induced percutaneously with or without guidance or more safely and efficiently intraoperatively under direct vision. Hsieh et al. (27) retrospectively compared 39 patients who underwent uniportal VATS anatomic lung resection for malignant and benign tumors who were treated with a bolus of levobupivacaine (10 mL, 0.5%) at the time of the chest closure combined with postoperative continuous infusion (0.5%, 2 mL/h) via a catheter placed at the ICS of the incision with 39 patients who also underwent uniportal anatomic lung resection but who were not offered ICNB. The authors found that that the ICNB group scored less in pain rating scales, better in Tri-flow performance and consumed less intravenous morphine. The effective analgesia achieved via the ICNB led to a shorter chest tube drainage and LOS for the ICNB group (22). Bolotin et al. prospectively randomized 32 patients who were submitted to uniportal VATS sympathectomy for palmar hyperhidrosis into a group (16 patients) which was offered ICNB immediately after the sympathectomy via a 3 cc/0.5% bupivacaine single-shot bupivacaine and into a control group which did not receive ICNB. The ICNB group reported less pain at 30 and 90 minutes postoperatively and at the discharge form the recovery room and consumed less pethidine. Rice et al. retrospectively matched two groups of patients (108 patients, 54 groups) and compared the result on postoperative pain between ICNB with a single-shot 2 cc/13.3 mg/mL liposomal bupivacaine injection and TEA with a combination of a local anesthetic (bupivacaine 0.075% or 1.0%) with hydromorphone or fentanyl. Both groups reported low pain scores without significant difference between them. Moreover, although there were no differences in the hypotension rate or the nadir systolic blood pressure (SBP), a patient in the TEA group had a dural puncture which resulted into cerebrospinal fluid leakage. Interestingly the ICNB group had a significantly postoperative LOS. The authors implied that the techniques are equally effective in postoperative pain management but noted the potentially serious complications with TEA. They also stressed out the prolonged effect of liposomal bupivacaine (4 days) which makes the use of an intercostal catheter for continuous infusion unnecessary (27-29).
In uniportal VATS, unlike other multi-port techniques, the injury is limited to a single intercostal nerve, which could allow an even less invasive anesthetic approach, without the use of TEA. There is evidence coming from cases operated on via non-intubated uniportal VATS, where an ICNB of the ICS of the incision was used as the mere method of regional anesthesia (30).
SAB
This technique, recently described by Blanco et al., involves the injection of a local anesthetic agent in the plane between the serratus anterior and the latissimus dorsi and between the chest wall and the serratus anterior, at the level of the fifth rib. The authors claim that the effect of SAB can last up to 750–840 minutes and can block dermatomes corresponding to levels T2–T9 (31). Ökmen et al. demonstrated that when SAB (20 mL 0.25% bupivacaine single-shot infusion) is added to the postoperative pain management protocol, patients experience significantly less pain and consume significantly less opioids after thoracotomy (32). Khalil et al. randomized 40 patients who underwent pulmonary resection via thoracotomy into a SAB and TEA group. The SAB group was offered a bolus dose of 30 mL 0.25% levobupivacaine followed by continuous infusion of 0.125% levobupivacaine (5 mL/h) while in the TEA group a bolus dose of 15 mL 0.25% levobupivacaine at the end of the surgery was followed by a 5 mL/h 0.125% levobupivacaine continuous infusion. The two groups demonstrated similar pain scores and 24 h morphine consumption. However, in the TEA group a significant drop in SBP occurred postoperatively compared to preoperative baseline levels. Five patients in the TEA necessitated epinephrine use to restore their blood pressure compared to none in the SAB group. The authors concluded that SAB provides efficient post-thoracotomy pain control, equal to that of TEA, but it lacks adverse events such as hypotension (33). Shariat used SAB as a primary anesthetic to drain a high-risk patient’s (receiving antiplatelet drug) right pleural cavity with single port VATS. Although the procedure was of minor complexity and short duration and did not involve manipulation of the lung or stimuli to the parietal pleura (apart from the initial incision), the author emphasized on the fact that SAB can be safely utilized in patients receiving anticoagulants, while TEA and PVB are contraindicated (34).
TEA
Thoracic epidural anesthesia has been the mainstay of postoperative pain management after thoracotomy. Via a catheter, opioids and local anesthetics can be infused into the epidural space either as a single-shot, or continuously via patient-controlled pumps. Although, in the era of minimally invasive procedures and despite the risk for adverse events, its role in the management of postoperative pain remains undisputed. Moreover, if opioids are omitted, adverse reactions, such as hypotension, vomiting and urinary retention can be avoided, thus making it possible even for enhanced-recovery regimens. In their prospective study, Obuchi et al. demonstrated that continuous, postoperative infusion of ropivacaine (0.2%, 2–6 mL/h) in the epidural space in patients undergoing thoracic procedures via an open approach has as a result similar pain scores with patients operated on via VATS (35). Even amongst patients undergoing VATS procedures those who are offered epidural analgesia, exhibit less postoperative pain when treated postoperatively with a single shot of 5 mL 0.25% bupivacaine combined with continuous infusion of 80 mL 0.25% bupivacaine and 1mg fentanyl citrate at a rate of 2 mL/h compared to those who are not (36).
Non-intubated uniportal VATS
Recently, the emerging of non-intubated VATS, has made it possible for patients to be operated on without the need for endotracheal intubation. The indications for such an approach are not clearly established but could include patients to whom general anesthesia could add significant morbidity. Non-intubated anesthetic techniques along with VATS and particularly uniportal (which induces the least surgical trauma) could also be implemented as part of an ERAS program as they represent the least invasive procedure, in terms of both anesthetic management and surgical trauma. In a non-intubated procedure, the patient breaths spontaneously, while the collapse of the non-depended lung due to the surgically induced pneumothorax provides a satisfactory view of the operating field. The procedure is carried out with the combination of one of the aforementioned regional anesthesia techniques and sedation. The sedation can vary, depending on the procedure and the surgical-anesthesia team’s preference from no sedation (BIS 90–100) to general anesthesia (BIS ≤60) (37). However, a highly skilled and vigilant anesthetist is required in order to ensure that the ventilation of the patient is not compromised.
The ventilation of patients undergoing non-intubated VATS is realized with the use of nasal prongs, simple face masks and supraglottic devices such as nasopharyngeal tube, the Guedel cannula and laryngeal masks. The range of thoracic operations feasible with non-intubated anesthesia is wide and includes both minor (treatment of pneumothorax, lung biopsies, wedge resections) and major ones (anatomic pulmonary resections). Minor procedures cam be carried out with one of the regional anesthesia techniques described above, or a combination of them, without the need for sedation, which is essential when conducting anatomic pulmonary resections. These require traction of the lung and maneuvers at the hilum which provide a strong stimulus for coughing. This stimulus cannot be suppressed by regional anesthetic techniques; thus, the need of sedation is mandatory (30).
Rocco et al. reported a uniportal VATS wedge resection of the middle lobe, conducted for diagnostic purposes. An epidural single shot of 1% ropivacaine solution at the level T5–6 was used for regional anesthesia and was supplemented by mild sedation via propofol. Fentanyl and midazolam were also administered (38). Gonzalez-Rivas et al. performed the first non-intubated uniportal VATS lobectomy. The patient was sedated via sevoflurane gas and continuous intravenous (IV) infusion of remifentanil. The skin was pre-emptively infiltrated with levobupivacaine. ICNB, again with levobupivacaine, was used as a means of regional anesthesia. Oxygen supplementation and airway control were established with the use of laryngeal mask. Vagus blockade and lidocaine spray of the lung were not necessary (39). The authors emphasized on the effective regional anesthesia with merely the infiltration of the ICS of the incision, the excellent collapse of the non-depended lung, the transient hypercapnia which was resolved quickly after the operation (in contrast with patients submitted to general anesthesia when hypercapnia may persist), the complete suppression of the cough reflex during the operation, achieved with target-controlled remifentanil infusion, the absence of throat soreness and the rapid return to preoperative physical condition (effective coughing minutes after the procedure, early oral intake and immediate mobilization). Chen et al. in his series of anatomic and non-anatomic non-intubated multiport VATS lung resections in 285 cases, reported favorable outcomes with a low rate of conversion to general anesthesia (4.9%), and commented on the need for vagus blockade (0.25% 2 mL bupivacaine) in order to suppress the cough reflex. Vagus blockade in this series was not found to affect the heart rate, the respiratory rate or the blood pressure. His anesthetic management also included the use of TEA (40). Other ways to suppress the cough, include spraying the lung with lidocaine, blocking the stellate ganglion or the vagus nerve as it courses the neck (41,42).
Case reports and case series, as the aforementioned, already available in the literature report promising results. A meta-analysis by Deng et al. consisting of 4 randomized control trials and ten observational studies concluded that patients submitted to non-intubated VATS for various procedures, obtained a significantly shorter global in-operating room time, hospital stay, lower complications rate and lower perioperative mortality (43). Although this meta-analysis contained a limited number of trials with a small sample size, it clearly documented the safety and efficacy of the technique and its, at least non-inferior, results compared to intubated VATS procedures.
Careful selection of patients and an experienced surgical and anesthesiology team are prerequisites for the success of any non-intubated VATS procedure. Also, the planned procedure should generally not be too long (44). Contraindications include patients with difficult airway, hemodynamically unstable, obese patients [body mass index(BMI) >30], coagulopathy [international normalized ratio (INR) >1.5], persistent cough and secretions, neurologic disorders which pose a risk for seizures or render patients unable to cooperate, intracranial mass, hypoxemia (PaO2 <60) or hypercapnia (PaCO2 >50). Also, a need to protect the contralateral lung from contamination (blood, pus, alveolar proteinosis) is prohibitory for a non-intubated procedure as well as any contradiction to receive regional anesthesia. Adhesions are not necessarily an absolute contraindication, but rather depend on the surgeon’s preference. It is well understood that a non-intubated VATS procedure should not be attempted by an inexperienced surgical and anesthesia team.
Conclusions
Uniportal VATS is a minimally invasive technique currently, established to treat benign and malignant diseases of the lungs, mediastinum, pleura diaphragm and esophagus via a single intercostal incision. The site of the incision varies depending on the planned procedure. Generally, for pulmonary resections the fifth ICS provides the more convenient access, although in special occasions the fourth can be utilized. For anterior mediastinal surgery the fourth ICP is indicated if dissection above the level of the innominate vein is required. Posterior mediastinal masses can be accessed via the fourth to sixth ICP depending on the expansion of the mass.
Up to now there is no consensus published regarding the most efficient way for the anesthetic management of patients treated via uniportal VATS. There is, however, a trend to apply a multimodality approach, including a combination of the aforementioned techniques combined with oral or IV analgesics in order to reduce the postoperative consumption of opioids which are related with adverse effects, such as nausea, vomiting, urine retention, and hallucination, that can hinder the recovery of the patient and prolong his hospitalization. In order to clarify the utility of each of the postoperative management techniques in the context of uniportal VATS, it is essential that multicenter randomized controlled and blinded studies are conducted.
Regarding non-intubated VATS, it seems that there is a variety of protocols regarding the selection of ventilation, the regional anesthesia, the sedation and the nerve blockades. Case series, case reports and meta-analyses give evidence of safety and efficacy as far as short-term results are concerned. but still more studies are required to further validate its outcomes, as well as evaluate the long-term outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yang X, Li M, Yang X, et al. Uniport versus multiport video-assisted thoracoscopic surgery in the perioperative treatment of patients with T1–3N0M0 non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2018;10:2186-95. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal Video-Assisted Thoracoscopic Lobectomy: Two Years of Experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Wu CF, Diego GR, Wen CT, et al. Single-port video-assisted thoracoscopic mediastinal tumour resection. Interact Cardiovasc Thorac Surg 2015;21:644-9. [Crossref] [PubMed]
- Stamenovic D. New technique of diaphragmatic plication by means of uniportal video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2017;25:162-3. [Crossref] [PubMed]
- Dmitrii S, Pavel K. Uniportal Video-Assisted Thoracic Surgery Esophagectomy. Thorac Surg Clin 2017;27:407-15. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Chen PR, Chen CK, Lin YS, et al. Single-incision thoracoscopic surgery for primary spontaneous pneumothorax. J Cardiothorac Surg 2011;6:58. [Crossref] [PubMed]
- Ismail M, Nachira D, Meacci E, et al. Uniportal video-assisted thoracic surgery in the treatment of pleural empyema. J Thorac Dis 2018;10:S3696-703. [Crossref] [PubMed]
- Chen YB, Ye W, Yang WT, et al. Uniportal versus biportal video-assisted thoracoscopic sympathectomy for palmar hyperhidrosis. Chin Med J 2009;122:1525-8. [PubMed]
- Gonzalez-Rivas D, Sihoe ADL. Important technical details during Uniportal Video-Assisted Thoracoscopic major resections. Thorac Surg Clin 2017;27:357-72. [Crossref] [PubMed]
- Sihoe AD. Uniportal video-assisted thoracoscopic lobectomy. Ann Cardiothorac Surg 2016;5:133-44. [Crossref] [PubMed]
- Sihoe AD, Chawla S, Paul S, et al. Technique for delivering large tumors in video-assisted thoracoscopic lobectomy. Asian Cardiovasc Thorac Ann 2014;22:319-28. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Videoassisted thoracic surgery lobectomy report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Wu CF, de la Torre M. Uniportal video-assisted thoracoscopic thymectomy and resection of a giant thymoma in a patient witness of Jehova. J Thorac Dis 2017;9:E556-9. [Crossref] [PubMed]
- Bayman EO, Kalpaj Parekh R, Keech J, et al. A Prospective Study of Chronic Pain after Thoracic Surgery. Anesthesiology 2017;126:938-51. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Song N, Zhao DP, Jiang L, et al. Subxiphoid uniportal video-assisted thoracoscopic surgery (VATS) for lobectomy: a report of 105 cases. J Thorac Dis 2016;8:S251-7. [PubMed]
- Zieliński M, Gwozdz P, Solarczyk-Bombik K, et al. Video-assisted thoracic surgery thymectomy: subxiphoid approach. Mediastinum 2018;2:45. [Crossref]
- Ko R, Mcrae K, Darling G, et al. The use of air in the inspired gas mixture during two-lung ventilation delays lung collapse during one-lung ventilation. Anesth Analg. 2009;108:1092-6. [Crossref] [PubMed]
- Gao S, Zhang Z, Brunelli A, Chen C, et al. The Society for Translational Medicine: clinical practice guidelines for mechanical ventilation management for patients undergoing lobectomy. J Thorac Dis 2017;9:3246-54. [Crossref] [PubMed]
- Zhu Y, Liang M, Wu W, et al. Preliminary results of single port versus triple-port complete thoracoscopic lobectomy for non-small cell lung cancer. Ann Transl Med 2015;3:92. [PubMed]
- Sihoe AD, Manlulu AV, Lee TW, et al. Pre-emptive local anesthesia for needlescopic video-assisted thoracic surgery: a randomized controlled trial. Eur J Cardiothorac Surg 2007;31:103-8. [Crossref] [PubMed]
- Fiorelli A, Vicidomini G, Laperuta P, et al. M.Pre-emptive local analgesia in video-assisted thoracic surgery sympathectomy. Eur J Cardiothorac Surg 2010;37:588-93. [Crossref] [PubMed]
- Wu Z, Fang S, Wang Q, et al. Patient-Controlled Paravertebral Block for Video-Assisted Thoracic Surgery: A Randomized Trial. Ann Thorac Surg 2018;106:888-94. [Crossref] [PubMed]
- Komatsu T, Kino A, Inoue M, et al. Paravertebral block for video-assisted thoracoscopic surgery: analgesic effectiveness and role in fast-track surgery. Int J Surg 2014;12:936-9. [Crossref] [PubMed]
- Hsieh MJ, Wang KC, Liu HP, et al. Management of acute postoperative pain with continuous intercostal nerve block after single port video assisted thoracoscopic anatomic resection. J Thorac Dis 2016;8:3563-71. [Crossref] [PubMed]
- Bolotin G, Lazarovici H, Uretzky G, et al. The efficacy of intraoperative internal intercostal nerve block during video-assisted thoracic surgery on postoperative pain. Ann Thorac Surg 2000;70:1872-5. [Crossref] [PubMed]
- Rice DC, Cata J, Mena G, et al. Posterior Intercostal Nerve Block with Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. Ann Thorac Surg 2015;99:1953-60. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia 2013;68:1107-13. [Crossref] [PubMed]
- Ökmen K, Metin Ökmen B. Evaluation of the effect of serratus anterior plane block for pain treatment after video-assisted thoracoscopic surgery. Anaesth Crit Care Pain Med 2018;37:349-53. [Crossref] [PubMed]
- Khalil AE, Abdallah NM, Bashandy GM, et al. Ultrasound-guided serratus anterior plane block versus thoracic epidural analgesia for thoracotomy pain. J Cardiothorac Vasc Anesth 2017;31:152-8. [Crossref] [PubMed]
- Shariat A, Bhatt H. Successful Use of Serratus Plane Block as Primary Anesthetic for Video-Assisted Thoracoscopic Surgery (VATS)-Assisted Pleural Effusion Drainage. J Cardiothorac Vasc Anesth 2018;32:e31-2. [Crossref] [PubMed]
- Obuchi T, Yoshida Y, Moroga T, et al. Postoperative pain in thoracic surgery: re-evaluating the benefits of VATS when coupled with epidural analgesia. J Thorac Dis 2017;9:4347-52. [Crossref] [PubMed]
- Yoshioka M, Mori T, Kobayashi H. The efficacy of epidural analgesia after video-assisted thoracoscopic surgery: a randomized control study. Ann Thorac Cardiovasc Surg 2006;12:313-8. [PubMed]
- Irons JF, Martinez G. Anaesthetic considerations for non-intubated thoracic surgery. J Vis Surg 2016;2:61. [Crossref] [PubMed]
- Rocco G, Romano V, Accardo R, et al. Awake single-access (uniportal) video-assisted thoracoscopic surgery for peripheral pulmonary nodules in a complete ambulatory setting. Ann Thorac Surg 2010;89:1625-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fernandez R, de la Torre M, et al. Single-port thoracoscopic lobectomy in a nonintubated patient: the least invasive procedure for major lung resection? Interact Cardiovasc Thorac Surg 2014;19:552-5. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Guo Z, Shao W, Yin W, et al. Analysis of feasibility and safety of complete video-assisted thoracoscopic resection of anatomic pulmonary segments under non-intubated anesthesia. J Thorac Dis 2014;6:37-44. [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [Crossref] [PubMed]
- Deng HY, Zhu ZJ, Wang YC, et al. Non-intubated video-assisted thoracoscopic surgery under loco-regional anaesthesia for thoracic surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2016;23:31-40. [Crossref] [PubMed]
- Kao MC, Lan CH, Huang CJ. Anesthesia for awake video-assisted thoracic surgery. Acta Anaesthesiol Taiwan 2012;50:126-30. [Crossref] [PubMed]