
Prognostic significance of preoperative lymph node assessment for patients with stage pN0 esophageal squamous cell carcinoma after esophagectomy
Introduction
Esophageal cancer is one of the most common aggressive malignancies worldwide, causing more than 400,000 deaths each year (1) with overall 5-year rate of 20% (2,3). Esophageal squamous cell carcinoma (ESCC) is the predominant histological type, accounts for about 90% of esophageal carcinoma cases (3). So far, surgical resection is still the most effective treatment for esophageal cancer (4). And regional lymph nodes (LNs) involvement status is considered to be one of the most reliable prognostic factors for patients with ESCC (5). Therefore, precise lymph node evaluation before surgical treatment is important to choose proper clinical plan and estimate the prognosis of patients. For ESCC patients accepting esophagectomy, many staging methods can be used, such as chest computed tomography (CT), endoscopic ultrasonography (EUS) and positron emission tomography (PET). Although several reports have compared the performance of these imaging methods in preoperative staging, the accuracy and validity of regional LNs assessment is still controversial (6,7). Compared with EUS and PET, the non-invasive property and cost efficacy enable CT as the most commonly used method in mediastinal LN evaluation. It has been reported that CT had a sensitivity of 30–60% and a specificity of 60–80% for identifying enlarged lymph nodes (8), and the accuracy of CT in N staging can be 46–58% (9). It means that preoperative clinical N staging and final pathological evaluation are occasionally discordant. The accuracy of postoperative evaluation of N staging may be affected by the quality of lymphadenectomy. For patients with clinical positive but pathological negative LN status, there is the possibility that pathological N stage may be underestimated because of the insufficient of LN dissection.
The aim of this study is to observe whether preoperative LN status evaluated by CT scan can affect the prognosis of stage pN0 ESCC patients. Besides, we proposed to discuss the different strategy of LNs dissection for patients with different LN status evaluated by preoperative CT scan.
Methods
Patients population
We consecutive retrospectively collected patients who underwent radical esophagectomy in the Department of Thoracic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine, during the period of 2009 to 2016. Patients with pathologically confirmed pN0 ESCC were enrolled in our study. The exclusion criteria were as follow: (I) patients with esophageal adenocarcinoma or other diseases; (II) tumors of the gastroesophageal junction; (III) patients with positive surgical margin; (IV) distant metastasis; (V) patients with perioperative deaths. All patients didn’t receive any radiation therapy or chemotherapy before the surgery. This study was conducted with approval from the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Reference Number 20181016).
Preoperative assessment of LNs by CT scan
All patients had received enhanced chest CT examination before surgery. Lymph nodes status evaluation was obtained mainly using enhanced CT images because few patients received EUS or PET-CT. Preoperative diagnosis of LN status was made by seasoned radiologists and surgeons. According to the CT scan reports, all patients were divided into two groups: CT positive group and CT negative group. Lymph nodes with the shortest diameter <5 mm were considered to be in normal size (CT negative group). Patients with suspected enlargement (5–10 mm) and definite enlargement (>10 mm) lymph nodes were both classified as CT positive group in this study (10) (Figure 1).
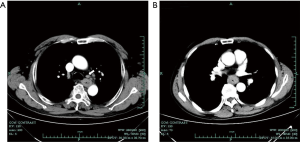
Surgery procedure and post-surgical follow-up
Radical esophagectomy was performed via a Sweet, Ivor-Lewis or Mckeown procedure with lymphadenectomy. The preferred substitute for the esophagus was an elevated gastric tube. LN sites were identified by surgeons, and the numbers of harvested LNs were recorded postoperatively. Histopathological examination of all resected specimens was performed by experienced pathologists, including the evaluation of tumor size, T stage, grading, resection margin. All dissected lymph nodes were microscopically analyzed for metastatic disease. The pathological staging was conducted according to the American Joint Commission on Cancer/Union for International Cancer Control (AJCC/UICC 8th version) tumor-node-metastasis (TNM) staging system (11).
All patients were regularly followed-up by outpatient clinic or telephone. The last follow-up time was January 2018.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics, version 20.0 (SPSS Inc, Chicago, IL). Overall survival (OS) was calculated using Kaplan-Meier curves, survival differences between groups were analyzed using log-rank test. The univariate and multivariate Cox proportional hazards regression models were used to evaluate the survival difference among groups, with hazard ratio (HR) and 95% confidence intervals (95% CI) provided. For all of the analysis, two-side P value less than 0.05 was considered to be statistically significant. And X-tile analysis was used to determine the thresholds for the number of harvested LNs with minimal P value (12).
Results
Clinicopathologic characteristics
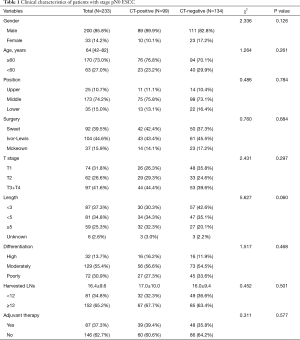
A total of 233 patients with stage pN0 ESCC who underwent radical esophagectomy were enrolled in this study. Among these patients, the median age was 64 years (range, 42–82 years), and 200 (85.8%) were male and 33 (14.2%) were female. Most tumors (74.2%) originated from the middle thoracic esophagus. Based on the 8th AJCC staging system, 74 patients (31.8%) were classified as T1 stage, 62 patients (26.6%) as T2 stage, 97 patients (41.6%) as T3 or T4 stage. The median harvested LNs was 15 (range, 2–49). Clinical characteristics are shown in Table 1.
Full table
According to preoperative CT scan performance, 99 patients (42.5%) were considered as LN metastasis positive and 134 (57.5%) as LN metastasis negative. And there were no significant differences in clinicopathologic characteristics between CT positive group and CT negative group (Table 1).
Survival analysis of patients with stage pN0 ESCC
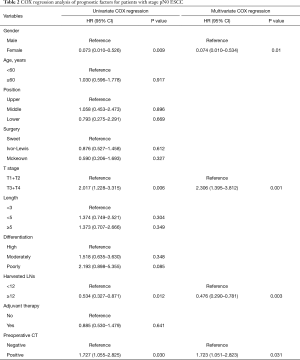
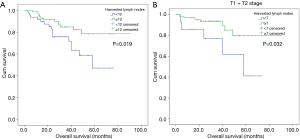
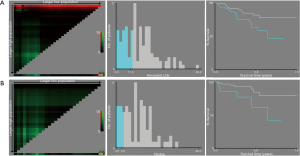
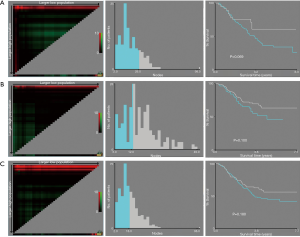
Univariate analysis showed that gender (HR: 0.073; 95% CI: 0.010–0.526; P=0.009), T stage (HR: 2.017; 95% CI: 1.228–3.315, P=0.006), harvested lymph nodes (HR: 0.534; 95% CI: 0.327–0.871; P=0.012) and preoperative LN status on CT scan (HR: 1.727; 95% CI: 1.055–2.825; P=0.030) were associated with OS of patients with pN0 stage ESCC (Table 2). And X-tile analysis indicated the cut-off value of dissected LNs should be 12 (Figure S1). Kaplan-Meier curves were showed in Figure 2. When preoperative CT scan indicated LNs metastasis, patients were more likely to have poor prognosis, even though they were all classified as N0 stage confirmed by postoperative pathology (P=0.027) (Figure 2). And the number of harvested LNs ≥12 also had a strongly positive influence on OS (P=0.010) (Figure 2).
Full table
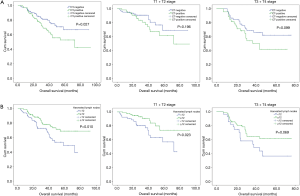
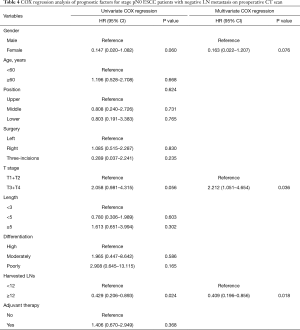
Multivariate COX regression analysis indicated that gender (HR: 0.074; 95% CI: 0.010–0.534; P=0.01), T stage (HR: 2.306; 95% CI: 1.395–3.812; P=0.001), harvested LNs (HR: 0.476; 95% CI: 0.290–0.781; P=0.003) and preoperative CT scan performance (HR: 1.723; 95%CI: 1.051–2.823; P=0.031) were all independent prognostic factors for patients with pN0 stage ESCC (Table 2).
Survival analysis for stage pN0 ESCC patients with positive N metastasis on preoperative CT scan
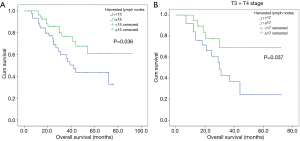
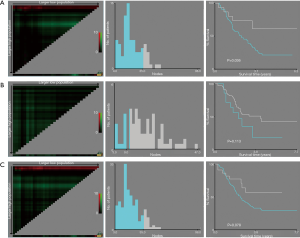
Ninety-nine patients were considered to have LN metastasis on preoperative CT scan but pathologically confirmed as pN0 stage. X-tile analysis indicated that for ESCC patients who were suspected to have LN metastasis on CT scan but pathologically confirmed as stage pN0, when the number of LNs dissected ≥15, they could significantly have better prognosis (P=0.036) (Figure S2). Kaplan-Meier curves were depicted in Figure 3. And we also found that for patients with higher T stage (stage T3 or T4), when at least 17 LNs was dissected, they were supposed to obtain survival benefits (P=0.037) (Figure 3, Figure S2).
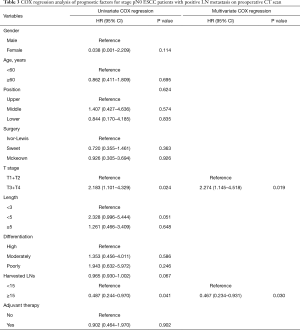
Univariate COX regression analysis indicated that T stage (HR: 2.183; 95% CI: 1.101–4.329; P=0.024) and the number of harvested LNs (HR: 0.487; 95% CI: 0.244–0.970; P=0.041) were significantly related to the prognosis of patients (Table 3). Multivariable also showed that T stage (HR: 2.274; 95% CI: 1.145–4.518; P=0.019) and the number of harvested LNs (HR: 0.467; 95% CI: 0.234–0.931; P=0.030) were both independent prognostic factors (Table 3).
Full table
Survival analysis for stage pN0 ESCC patients with negative LN metastasis on preoperative CT scan
The consistency of preoperative and postoperative LN assessment was seen in 134 patients. Kaplan-Meier curves were showed in Figure 4. The number of LNs dissected ≥12 was a positive prognostic factor (P=0.019) for ESCC patients (Figure S3). It should be noted that for stage T1 or T2 ESCC patients without positive LN metastasis performance on preoperative CT scan, they could also have better clinical outcomes with LNs dissected equal to or more than 7 (P=0.032) (Figure 4, Figure S3).
Both univariate and multivariate COX regression analysis indicated that the number of harvested LNs were strongly related to the prognosis of patients (Table 4).
Full table
Discussion
In this study, we emphasized the prognostic significance of preoperative lymph node assessment for patients with pN0 ESCC receiving esophagectomy, and we firstly proposed that the optimal LN dissection number should refer to the preoperative CT performance, which could give precisely clinical guidance for the strategy of LN dissection.
Esophageal cancer is an aggressive malignancy with a high incidence of lymph nodes metastasis, as esophageal cancer typically spreads via the lymphatic system (13). The status of lymph nodes has been considered as the most critical prognostic factors affecting long term survival for patients with ESCC (14). And radical lymphadenectomy might be an important method to improve survival (14). Therefore, a meaningful decision-making and management of esophageal cancer requires an accurate preoperative staging. Esophageal CT scan is the most commonly used method for preoperative assessment. However, the accuracy of CT scan in N staging is unsatisfactory, because the diagnostic criterion of N metastasis is still controversial. An obvious limitation of CT in N staging involves metastasis without obvious enlargement in size, as well as the fact that enlarged nodes may contain no metastasis (15). The current standard of positive lymph node set the shortest diameter as 10 mm (16), but this standard is just a clinical estimated value without any pathological evidence. Recent studies indicated that the shortest diameter of diagnostic criteria of lymph nodes could be less than 10 mm on CT (17). Mizowaki et al. suggested that the criterion was ≥5 mm in the shortest diameter (10). In our study, we accepted Mizowaki’s criteria, lymph nodes with the shortest diameter ≥5 mm were considered as positive for metastasis, but the experience of radiologists and surgeons were also taken into account. However further research was needed to confirm the most reasonable diagnostic criteria.
We retrospectively analyzed 233 patients with stage pN0 ESCC who received radical esophagectomy during the period of 2009 to 2016. Among them, 99 patients (42.5%) were considered to have N metastasis assessed by preoperative CT scan but confirmed as N0 stage by postoperative pathological examination. In general, pathological N metastasis rather than clinical N metastasis was an independent prognostic factor for overall survival of patients with ESCC (18). Interestingly, we proved that patients with suspected clinical N metastasis had poorer prognosis (P=0.027). Cox regression analysis also indicated that preoperative clinical N staging was an independent prognostic factor for patients with stage pN0 ESCC. A previous SEER-based analysis indicated that patients classified as stage pN0 had fewer LNs harvested than those classified as stage N1 (19). It could be suspected that N staging might be underestimated due to the insufficient nodal dissection, especially for patients with suspected clinical N metastasis. Theoretically, resecting more lymph nodes or finding more negative lymph nodes may reduce the risk of occult lesions and thus increase the survival rate (20). Inadequate nodal dissection couldn’t reflect the real stage; however, overtreatment may result in increasing complications and mortality. Thus, the optimal and individualized lymph node dissection is important. Currently, there are still controversies about the exact number of harvested LNs in ESCC. Hu et al. suggested that more than 6 LNs should be resected for appropriate evaluation of LN status (21). Dutkowshi et al. believed that when the number of examined LNs ≥12, the accuracy for N staging could reach 90% (22). But there were other studies showed that the criteria of LN dissection should be 15 (23) or 18 (24). We found that for patients with CT-evaluated LN-negative ESCC, the number of harvested LNs was an independent prognostic factor, and the adequate number of examined LNs should be 12 (P=0.019). It should be noted that for patents with suspected clinical N metastasis on CT scan, when the number of harvested lymph nodes ≥15, patients could have better prognosis (P=0.036), especially for stage T3 or T4 patients, at least 17 LNs dissected were recommended for improved clinical outcomes (P=0.037).
However, this single-center retrospective study also had some limitations. The accurate judgement of positive LN metastasis on CT scans was still controversial and was tends to be driven more by experience, the validity of our criteria to assess LN status on CT scan was needed for further verification. And in order to determine the optimal number of LNs dissected by LN status based on preoperative CT evaluation, both histological positive and negative LN cases should to be analyzed. However, this part of data was showed in supplemental material (Figures S4,S5) and no significant results were obtained. We considered that the number of dissected lymph nodes was much more important for the confirmation of true pN0 stage. Insufficient number of dissected LNs may affect the accuracy of N staging, especially for the diagnosis of stage N0. So that our results may be helpful in the systematic dissection of lymph nodes and promote the accuracy of N staging. However, more researches are required to confirm the adequate number of dissected LNs according to preoperative LN status.
In conclusion, preoperative LN assessment for ESCC patients is critically important, the optimal number of dissected LNs should refer to the preoperative CT performance. For patients with suspect positive LN metastasis on CT scan, LN dissection should be much more careful and systematic, at least 15 LNs should be dissected, and especially for patients with higher T stage, at least 17 LNs dissected were recommended.
Acknowledgements
Funding: This work was supported by National Key R&D Program of China (2017YFC0113500), Traditional Chinese Medicine (Integrated Chinese and Western Medicine) Key Discipline of Zhejiang Province (2017-XK-A33).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was conducted with approval from the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Reference Number 20181016).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal cancer. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Winiker M, Mantziari S, Figueiredo SG, et al. Accuracy of preoperative staging for a priori resectable esophageal cancer. Dis Esophagus 2018;31:1-6. [Crossref] [PubMed]
- Schweigert M, Dubecz A, Stein HJ. Oesophageal cancer--an overview. Nat Rev Gastroenterol Hepatol 2013;10:230-44. [Crossref] [PubMed]
- Choi J, Kim SG, Kim JS, et al. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc 2010;24:1380-6. [Crossref] [PubMed]
- Pfau PR, Perlman SB, Stanko P, et al. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc 2007;65:377-84. [Crossref] [PubMed]
- Kato H, Kuwano H, Nakajima M, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 2002;94:921-8. [Crossref] [PubMed]
- Tio TL, Cohen P, Coene PP, et al. Endosonography and computed tomography of esophageal carcinoma. Preoperative classification compared to the new (1987) TNM system. Gastroenterology 1989;96:1478-86. [Crossref] [PubMed]
- Mizowaki T, Nishimura Y, Shimada Y, et al. Optimal size criteria of malignant lymph nodes in the treatment planning of radiotherapy for esophageal cancer: evaluation by computed tomography and magnetic resonance imaging. Int J Radiat Oncol Biol Phys 1996;36:1091-8. [Crossref] [PubMed]
- Rice TW, Kelsen D, Blackstone EH, et al. Esophagus and Esophagogastric Junction. In: AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017:185-202.
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Akutsu Y, Matsubara H. Lymph node dissection for esophageal cancer. Gen Thorac Cardiovasc Surg 2013;61:397-401. [Crossref] [PubMed]
- Kelty CJ, Kennedy CW, Falk GL. Ratio of metastatic lymph nodes to total number of nodes resected is prognostic for survival in esophageal carcinoma. J Thorac Oncol 2010;5:1467-71. [Crossref] [PubMed]
- Luo LN, He LJ, Gao XY, et al. Evaluation of preoperative staging for esophageal squamous cell carcinoma. World J Gastroenterol 2016;22:6683-9. [Crossref] [PubMed]
- Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features of endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc 1997;45:474-9. [Crossref] [PubMed]
- Li J, Chen S, Zhu G. Comparative study of computed tomography (CT) and pathological diagnosis toward mediastinal lymph node metastasis in esophageal carcinoma. Rev Assoc Med Bras (1992) 2018;64:170-4. [Crossref] [PubMed]
- Yokota T, Ando N, Igaki H, et al. Prognostic factors in patients receiving neoadjuvant 5-fluorouracil plus cisplatin for advanced esophageal cancer (JCOG9907). Oncology 2015;89:143-51. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Clinical Impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg 2007;11:1384-93. [Crossref] [PubMed]
- Wu SG, Li FY, Zhou J, et al. Prognostic value of different lymph node staging methods in esophageal squamous cell carcinoma after esophagectomy. Ann Thorac Surg 2015;99:284-90. [Crossref] [PubMed]
- Hu Y, Hu C, Zhang H, et al. How does the number of resected lymph nodes influence TNM staging and prognosis for esophageal carcinoma? Ann Surg Oncol 2010;17:784-90. [Crossref] [PubMed]
- Dutkowski P, Hommel G, Böttger T, et al. How many lymph nodes are needed for an accurate pN classification in esophageal cancer? Hepatogastroenterology 2002;49:176-80. [PubMed]
- Barbour AP, Rizk NP, Gonen M, et al. Lymphadenectomy for adenocarcinoma of the gastroesophageal junction (GEJ): Impact of adequate staging on outcome. Ann Surg Oncol 2007;14:306-16. [Crossref] [PubMed]
- Yang HX, Xu Y, Fu JH, et al. An evaluation of the number of lymph nodes examined and survival for node-negative esophageal carcinoma: data from China. Ann Surg Oncol 2010;17:1901-11. [Crossref] [PubMed]


