
Low-dose indocyanine green fluorescence-navigated segmentectomy: prospective analysis of 20 cases and review of previous reports
Introduction
The frequency of detection of ground-glass nodule (GGN) and small pulmonary metastases has increased due to advances in clinical imaging/staging modalities. In early-stage non-small cell lung cancer (NSCLC), sublobar resection has shown a trend toward a more favorable prognosis than lobectomy (1,2). Lung segmentectomy is more efficient at securing a sufficient surgical margin than wedge resection. Traditionally, segmental lines were identified intraoperatively with inflation/deflation of the target segment by clamping and unclamping the relevant bronchus. However, the inflated lung may obstruct the view of the target segment in video-assisted thoracic surgery (VATS).
The technique of visualizing the demarcation line based on near-infrared (NIR) fluorescence imaging with indocyanine green (ICG) was recently developed, and its utility was reported (3-13). However, the route and dose of ICG injection have not been confirmed. In a previous study, the dose of ICG used for lung segmentectomy ranged from 0.25 to 5 mg/kg (3-6,8,12).
Therefore, in the present study, we demonstrated the safety and utility of low-dose (5 mg/body) ICG fluorescence-navigated VATS segmentectomy.
Methods
Study patients
This study was prospectively performed with data collected from patients who had undergone lung segmentectomy for early-stage NSCLC or small pulmonary metastasis at our hospital between March 2017 and August 2018. Patients were excluded if they were younger than 19 years of age or older than 85 years of age or had undergone lobectomy or wedge resection. This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review boards of our hospital approved the protocol (the approval number: M416), and written informed consent was obtained from all patients.
Surgical procedures
All patients underwent a preoperative evaluation by computed tomography (CT) scanning. The CT images were reconstructed three-dimensionally (3D) to evaluate the anatomy of the pulmonary vessels and bronchi and identify the dominant pulmonary arteries and veins of the target segment by image processing software (Ziostation 2, version 2.9.2.3, Ziosoft, Tokyo, Japan). We performed pulmonary segmentectomy by VATS. We called the surgery only for monitoring view as complete VATS (C-VATS), on the other hand, the surgery combined with direct vision, not rib spreading as hybrid VATS (H-VATS). The target pulmonary arteries and veins were ligated, and then the target bronchi were ligated. ICG (Daiichi Sankyo Co., Ltd., Tokyo, Japan) at 5 mg/body was rapidly injected into the peripheral vein, and the lung was observed using NIR fluorescence thoracoscopy. Under the NIR light, the demarcation line of the target segment was separated into two areas and then marked on the visceral pleura with electrocautery. In this study, we used four types of NIR thoracoscopy system: IMAGE1 STM, KARL STORZ, Endoscope (Japan K.K., Tokyo, Japan); PINPOINT Endoscopic Fluorescence Imaging System (NOVADAQ, Ontario, Canada); VISERA ELITE II (OLYMPUS, Hamburg, Germany); and 1588 AIM (Stryker, Tokyo, Japan). We evaluated the identification rate of the demarcation line based on NIR fluorescence imaging with ICG and the perioperative outcomes (e.g., safety, operation time, mortality, and length of postoperative stay).
Endpoints
The primary endpoint was the identification rate of the demarcation line of the target segment. The secondary endpoint was the perioperative outcome.
Statistical analysis
All statistical analyses were carried out with the JMP software program (Version 13.2; SAS Institute, Inc., Cary, NC, USA). A P value of <0.05 was considered to be statistically significant.
Results
Patients characteristics
The patient characteristics are described in Table 1. There were 10 male and 10 female patients, and their median age was 72 years (range, 37–80 years). The median Brinkman index, which is calculated by daily number of cigarettes × years, was 0 (range, 0–2,640), the median body surface area (BSA) was 1.55 m2 (range, 1.39–1.97 m2). The median predictive vital capacity (%VC) was 100.5% (range, 63.1–120.6%), the median forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio (FEV1%) was 74.7% (range, 55.2–118.5%), and the median predictive diffusing capacity for carbon monoxide (%DLCO) was 90.1% (range, 47.1–170.4%). Eighteen cases were primary lung cancers, and two were metastatic lung tumors. The tumors were mainly in the right lower lobe and left upper lobe.
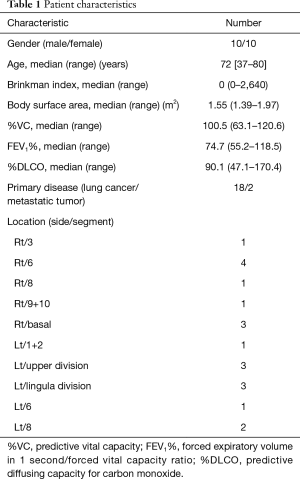
Full table
Perioperative data
The perioperative data are summarized in Table 2. Seven patients underwent C-VATS segmentectomy, 11 underwent H-VATS, and 2 underwent thoracotomy. For open thoracotomy, we used the VATS camera only when identify areas with ICG. The median operation time was 137.5 min (range, 82–175 min), and the median blood loss was 30 mL (range, 5–140 mL). The identification of the target segmental line was possible in 18 (90%) of the 20 patients. Although two patients were added ICG 5 mg because the target segmental line was unclear, the boundary line was unclear at all. Therefore, the segmental lines were identified intraoperatively with inflation/deflation of the target segment by clamping and unclamping the relevant bronchus for the two patients. Figure 1 shows that the segmental line is clearly identified using NIR fluorescence imaging with ICG. The median duration of chest tube placement was 1 day (range, 1–8 days), and the median length of postoperative stay was 8 days (range, 4–24 days). Complications occurred in 5 (25%) of the 20 patients. All cases were prolonged air leak that required pleurodesis. There were no complications resulting from NIR fluorescence imaging with ICG.
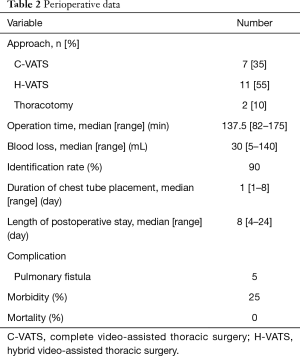
Full table
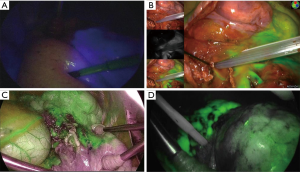
Discussion
Technological advances in imaging modalities, such as the advent of high-resolution CT, have resulted in the earlier detection of GGN or small metastatic lung tumor. In some reports, sublobar resection for early-stage NSCLC resection has shown a trend toward a more favorable prognosis than lobectomy (1,2). Furthermore, current evidence indicates that radical sublobar resection should be considered as an alternative for stage IA NSCLC of ≤2 cm, even in low-risk patients (14-16). It is important to identify the anatomic segmental line for lung segmentectomy. Traditionally, segmental lines were identified intraoperatively with inflation/deflation of the target segment by clamping and unclamping the relevant bronchus. However, the inflated lung may obstruct the view of the target segment, particularly in VATS. Several studies have reported the utility of identifying the segmental line using NIR fluorescence imaging with ICG (2-13). While the route of administration of ICG is mainly intravenous, intrabronchial administration has been attempted in a few cases. The identification rate of the demarcation line of the target segment with intravenous and intrabronchial administration ranged from 90–100% (Table 3). Although the utility of intrabronchial administration of ICG has also been reported, intravenous administration of ICG might be considered to be easier. ICG is generally considered safe, and the incidence of severe adverse reaction has been reported to be 0.05% (17). In previous studies, the dose of ICG used for lung segmentectomy ranged from 0.25 to 5 mg/kg (e.g., 15 to 300 mg of ICG applied to a patient weighing 60 kg), and no complications were attributed to ICG (3-6,8,12). However, the incidence of anaphylactic shock due to ICG used for angiography at doses of 25 to 75 mg was reported to be 0.05% (18). Furthermore, three cases of anaphylactoid reaction to ICG that may have been mediated by dose-dependent pseudo-allergic mechanism (19). Although the dose of ICG was quite low at 5 mg in the present study, the identification rate of the demarcation line of target segment was 90%, so this dose of ICG achieves a sufficient rate of identification of the segmental line. Using such a low dose of ICG is safe, as well, by helping avoid anaphylactic shock.

Full table
In this study, we used four types of NIR thoracoscopy system: IMAGE1 STM, KARL STORZ, Endoscope (Japan K.K.); PINPOINT Endoscopic Fluorescence Imaging System (NOVADAQ); VISERA ELITE II (OLYMPUS); and 1588 AIM (Stryker). Although we examined whether there were differences among these four NIR thoracoscopy systems, the segmental lines were clearly identified and there were no marked differences in the visualization of the target segmental line.
Limitations
Several limitations associated with the present study warrant mention. First, the study was performed in a small number of patients with a single-institution experimental design. Second, we failed to identify the segmental line in two cases. The blood flow of the emphysematous and bullous lung tissue is lower than that of the normal lung, so the visualization of the segmental line by ICG can be difficult in emphysematous lungs (20). However, while these two cases were indeed heavy smokers and had emphysematous lungs, other patients with emphysematous lungs showed well-defined borders. Therefore, the reason for the unclear visualization of the segmental line by ICG should be further explored in future studies.
In conclusion, NIR fluorescence imaging with ICG is safe and useful for the identification of the demarcation line for lung segmentectomy. Low-dose ICG might achieve a sufficient rate of identification of the segmental line while still avoiding anaphylactic shock.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review boards of Kanazawa Medical University approved the protocol (the approval number: M416), and written informed consent was obtained from all patients.
References
- Berfield KS, Wood DE. Sublobar resection for stage IA non-small cell lung cancer. J Thorac Dis 2017;9:S208-10. [Crossref] [PubMed]
- Sakurai H, Asamura H. Sublobar resection for early-stage lung cancer. Transl Lung Cancer Res 2014;3:164-72. [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013;44:1103-7. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Iizuka S, Kuroda H, Yoshimura K, et al. Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis 2016;8:985-91. [Crossref] [PubMed]
- Kuroda H, Dejima H, Mizumo T, et al. A new LigaSure technique for the formation of segmental plane by intravenous indocyanine green fluorescence during thoracoscopic anatomical segmentectomy. J Thorac Dis 2016;8:1210-6. [Crossref] [PubMed]
- Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. [Crossref] [PubMed]
- Ito A, Takao M, Shimamoto A, et al. Prolonged intravenous indocyanine green visualization by temporary pulmonary vein clamping: real-time intraoperative fluorescence image guide for thoracoscopic anatomical segmentectomy. Eur J Cardiothorac Surg 2017;52:1225-6. [Crossref] [PubMed]
- Yamanashi K, Okumura N, Nakazono C, et al. Surgery for Intralobar Pulmonary Sequestration Using Indocyanine Green Fluorescence Navigation: A Case Report. Semin Thorac Cardiovasc Surg 2018;30:122-4. [Crossref] [PubMed]
- Uramoto H, Motono N. ICG easily detects not only the segmental plane, but also the course and blood distribution of the bronchial artery"case report Ann Med Surg (Lond) 2018;28:28-9. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Oh S, Suzuki K, Miyasaka Y, et al. New technique for lung segmentectomy using indocyanine green injection. Ann Thorac Surg 2013;95:2188-90. [Crossref] [PubMed]
- Okada M. Radical sublobar resection for lung cancer. Gen Thorac Cardiovasc Surg 2008;56:151-7. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Yoshikawa K, Tsubota N, Kodama K, et al. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg 2002;73:1055-8; discussion 1058-9. [Crossref] [PubMed]
- Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529-33. [Crossref] [PubMed]
- Obana A, Miki T, Hayashi K, et al. Survey of complications of indocyanine green angiography in Japan. Am J Ophthalmol 1994;118:749-53. [Crossref] [PubMed]
- Speich R, Saesseli B, Hoffmann U, et al. Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med 1988;109:345-6. [Crossref] [PubMed]
- Gotoh M, Yamamoto Y, Igai H, et al. Clinical application of infrared thoracoscopy to detect bullous or emphysematous lesions of the lung. J Thorac Cardiovasc Surg 2007;134:1498-501. [Crossref] [PubMed]


