
Disparities in the surgical management of early stage non-small cell lung cancer: how far have we come?
Introduction
Lung cancer is the leading cause of cancer associated mortality in the United States (1). Of the 180,000 patients diagnosed with NSCLC each year, approximately one-third are found to have early stage (I–II) disease on clinical evaluation and are potential candidates for curative-intent surgical resection (2-4). The role of surgery as the principle treatment for localized NSCLC is well established, with prior research consistently demonstrating superior long-term overall survival following surgery as compared to treatment with radiation therapy alone or no therapy. For these reasons, guidelines put forth by the American College of Chest Physicians and the National Comprehensive Cancer Network recommend surgical resection for all medically operable patients with stage I–II NSCLC (5,6). However, despite the rigorous clinical and oncologic basis for these guidelines, disparities in the receipt of surgery for the treatment of early stage NSCLC exist.
Perhaps the seminal study on this topic was published by Peter Bach and colleagues in The New England Journal of Medicine in 1999 (7). Using the Surveillance, Epidemiology, and End Results (SEER) program database, the authors found that black patients with stage I–II NSCLC experienced significantly lower overall 5-year survival rates as compared to white patients with similarly staged disease. Importantly, this survival discrepancy was not observed when comparing black and white patients that underwent resection, which suggests that the observed difference in overall survival was a consequence of unequal rates of surgery between these two groups. Among the many important implications of this work was that it not only highlighted the existence of racial disparities in survival within a nationally representative cohort of lung cancer patients, but it also reaffirmed the importance of delivering appropriate therapies as an actionable approach to mitigating these survival differences in practice.
Since Bach’s publication, the literature on disparities in the surgical management of lung cancer has grown substantially. To date, several studies have characterized treatment and outcome inequities in different racial, socioeconomic status, and other disadvantaged groups. Therefore, the central goal of this narrative review is to provide a contemporary examination of nearly 20 years of surgical disparities literature in the lung cancer population. Specifically, this study aims to summarize common and unrecognized disparities in the receipt of surgical resection for early stage NSCLC and the potential mechanisms that may perpetuate these disparities in clinical practice.
Methods
Data source
The MEDLINE (PubMed) database was queried to identify articles describing surgical disparities in the surgical management of early stage NSCLC published between January 1999 and October 2018. The following terms were used to guide this search strategy: “surgical disparities”, “treatment disparities”, or “disparities” combined with “early stage non-small cell lung cancer” or “lung cancer”. Manuscript abstracts were reviewed to determine inclusion eligibility as primary source references. We focused on reports that specifically evaluated disparities in the receipt of surgery or in the patient, physician, or health system factors potentially associated with the decision to pursue surgery for localized NSCLC. In addition to the articles identified using this methodology, we included other studies as necessary to provide sufficient background and to contextualize the summarized findings.
Results
Since 1999, there has been a proliferation of studies on disparities in the receipt of surgery for localized NSCLC. While the majority of these studies have been performed in United States lung cancer patient cohorts, assessments of treatment and outcome disparities in the NSCLC population have also been reported in other regions such as Asia, Western Europe, and the United Kingdom (8-12). Despite more homogeneous racial/ethnic patient cohorts, these studies have found patient and neighborhood socioeconomic status to be associated with surgical disparities in NSCLC patients. Together these reports demonstrate that similar trends in the unequal access to surgical care observed in the United States are also observed in the international NSCLC population. Below we summarize common and less recognized disparities in the surgical management of NSCLC, focusing on race/ethnicity, socioeconomic and insurance payer status, area of residence, and selective patient cohorts.
Common and unrecognized disparities in the receipt of surgery for early stage NSCLC
Race and ethnicity
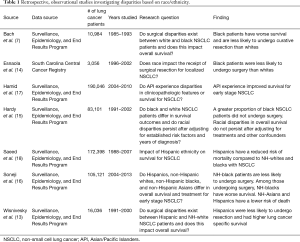
Bach and colleagues published one of the first major studies on racial disparities in the surgical management of early stage NSCLC in 1999 (7). Using the SEER program database, these authors observed that black patients were 12.7% less likely to undergo surgical resection for stage I–II NSCLC and experienced a significantly lower overall 5-year survival rate as compared to white patients with comparably staged disease (26.4% vs. 34.1%, P<0.001) (7). To emphasize the central findings of their work, the authors applied these results to a hypothetical cohort of 1,000 black and 1,000 white patients and demonstrated that up to 44 of the 77 additional deaths among black patients could be prevented with the appropriate delivery of surgical therapies. In the time since this publication, multiple groups have described similar observations in the black NSCLC population (13-16). Together, the cumulative data presented in these various institutional, regional, and national analyses suggest that black patients persistently experience inequity in the surgical management of NSCLC (Table 1).
Treatment disparities have also been described in other minority groups. In 2005, Wisnivesky et al. used the SEER database to study this question in the Hispanic population with stage I NSCLC (13). These authors found that Hispanic patients experienced lower rates of lung cancer-specific survival and surgical resection as compared to white patients (survival =54.2% vs. 64.2%, P=0.008; rate of resection =83% vs. 86%, P=0.03). Similar to the trends observed in the black population, after accounting for treatment received and stage at diagnosis, the racial differences in lung cancer-specific survival between Hispanic and white patients were no longer observed.
In 2017, Soneji et al. compared the use of surgery across four racial/ethnic groups (non-Hispanic whites, non-Hispanic blacks, Hispanics, and non-Hispanic Asians) with stage I–II NSCLC in the SEER database (16). After adjusting for other demographic and clinical factors, these authors found that non-Hispanic black patients experienced significantly worse overall survival as compared to non-Hispanic whites [adjusted hazard ratio (HR) =1.05; 95% CI: 1.02–1.08]. Among those who underwent surgery, the relative risk of death from lung cancer was similar between non-Hispanic black patients and whites. However, non-Hispanic blacks who received surgery did experience a higher relative risk of death from other causes as compared to white patients (adjusted RR =1.07; 95% CI: 1.02–1.12) (16). Notably, differences in the receipt of surgery were not observed in Hispanics or non-Hispanic Asians. After accounting for the treatment received, these groups experienced a lower risk of mortality as compared to white patients. While the exact reasons for the survival advantage among Hispanic and non-Hispanic Asian patients in this study were unclear, improved lung cancer survival among Asian patients has been reported in other studies as well (17,18).
American Indians and Alaskan Natives (AI/AN) together comprise a less commonly recognized racial minority group with disparate outcomes in the cancer literature. Smith et al. found that AI/AN SEER-Medicare lung cancer patients had more advanced disease at diagnosis and lower rates of lung cancer specific survival at 5-years (47%; 95% CI: 43.5–50.5%) as compared to non-Hispanic whites (56%; 95% CI: 55.7–56.3%), blacks (51%; 95% CI: 50.0–51.4%), Hispanic (55%; 95% CI: 53.8–56.2%), and other races/ethnicities (59%; 95% CI: 57.8–59.6%) (19). These authors determined that AI/AN patients were at increased risk of lung cancer associated morality as compared to white patients (HR =1.36; 95% CI: 1.15–1.62). As with previous minority groups, this difference was no longer observed after controlling for the stage at diagnosis and the use of surgery and/or radiation therapy (HR =1.17; 95% CI: 0.98–1.39), suggesting that underlying disparities in access to appropriate care may serve as the driving force behind the survival inequity among AI/AN patients. To this end, while Adams et al. reported that AI/AN SEER-Medicare patients do not experience significant delays in the receipt of lung cancer treatment, Javid et al. showed that AI/AN patients were less likely than whites to receive guideline-directed surgical and non-surgical care for the treatment of breast, colon, prostate, and lung cancer (20,21). Taken together, these data suggest that survival discrepancies in the AI/AN population are at least in part due to the failure to receive appropriate stage-specific therapies.
Full table
Socioeconomic and insurance payer status
While no consensus exists regarding a single metric to quantify socioeconomic status (SES), observational studies have frequently represented SES using variables such as median household income or occupational status. Given the relationship between these variables and health insurance, we examined SES in conjunction with insurance payer status (Table 2).
Full table
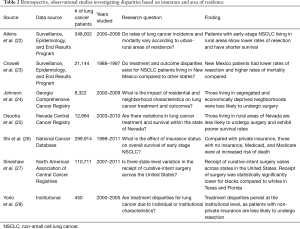
Shi et al. studied the effect of insurance payer status on survival for early stage NSCLC patients diagnosed between 1998 and 2011 in the National Cancer Database (NCDB) (26). When accounting for demographic, tumor, and clinical characteristics, Medicaid (HR =1.36; 95% CI: 1.31–1.42; P<0.0001), Medicare (HR =1.17; 95% CI: 1.15–1.20; P<0.0001), and uninsured patients (HR =1.21; 95% CI: 1.14–1.28; P<0.0001) had a greater hazard of death compared to those with private insurance. In addition to insurance type, Shi et al. observed that median household income was also significantly associated with survival. They reported an increased risk of mortality with each stepwise reduction in median household income (<$30,000: HR =1.16, 95% CI: 1.13–1.20, P<0.0001; $30,000–34,000: HR =1.09, 95% CI: 1.06–1.12, P<0.0001; $35,000–$45,000: HR =1.07, 95% CI: 1.05–1.09, P<0.0001), but did not include survival curves segregated according to these income strata. This work supports the hypothesis that socioeconomic and insurance payer status impacts survival in the NSCLC population, however, the authors did not evaluate whether these same factors were associated with the receipt of surgery, as has been reported in other state and institutional series.
In a study using stage I–II patients enrolled in the South Carolina state registry, Esnaola et al. showed that patients living in poverty had significantly lower odds of undergoing resection compared to those with higher incomes (OR =0.84; 95% CI: 0.72–0.99; P=0.038) (14). Insurance status was also found to be a significant covariate in their model, as patients with self-pay (OR =0.32; 95% CI: 0.21–0.48; P<0.0001), Medicaid (OR =0.27; 95% CI: 0.17–0.43; P<0.0001), Medicare (OR =0.31; 95% CI: 0.24–0.38; P<0.0001), or HMO insurance types (OR =0.50; 95% CI: 0.29–0.85; P=0.011) had increased odds of not receiving surgery as compared to those with private insurance.
Johnson et al. investigated the impact of residential and neighborhood characteristics on lung cancer treatment and outcomes using the Georgia Comprehensive Cancer Registry (24). To more comprehensively represent socioeconomic status, the authors constructed an “economic deprivation” variable. This composite metric was calculated based on the percentage of households that were: (I) below the poverty line, (II) had a female head of house and children, (III) on public assistance, and (IV) had non-married owners. The authors classified economic deprivation by quartile intervals for their analysis, and found that as economic deprivation increased, the receipt of surgery declined. These trends were independently observed in both black and white patients.
The impact of insurance status on treatment has also been demonstrated in institutional studies. In a cohort of 247 patients with resectable stage I–II NSCLC treated at an academic center in Texas, Yorio et al. found that patients with Medicaid or public county insurance (OR =0.13; 95% CI: 0.04–0.43) were significantly less likely to undergo resection than those with private insurance (28). When controlling for treatments rendered, this group did not demonstrate increased risk of mortality.
Area of residence (urban-rural status and geography)
In addition to disparities based on patient demographics, inequity due to residential and geographic region has been reported (Table 2). Atkins et al. published a comprehensive study on the impact of urban-rural area of residence on disease incidence, treatment, and outcomes in the SEER population (22). These investigators stratified patients according to nine rural-urban continuum (RUCA) codes abstracted from county-level population estimates ranging from less than 2,500 (most rural) to over 1 million (most urban). They found that increased rurality positively correlated with annual lung cancer incidence and associated mortality. For patients with stage I NSCLC, those living in the most rural areas experienced significantly lower median overall survival rates and were less likely to undergo surgical resection as compared to patients residing in the most urban regions (survival 38.5 vs. 52 months, P=0.0006; rate of surgery 69% vs. 75%). While there was no reported difference in the receipt of radiation therapy, rural patients were more likely to undergo no treatment compared to urban patients (17.6% vs. 13.2%; OR =1.40; P=0.007).
Rural disparities have also been observed at the regional and state levels. In 2007, Crowell et al. published a study comparing treatment and outcome patterns for urban and rural patients from New Mexico to those from eight other SEER state registries, including California, Connecticut, Detroit, Hawaii, Iowa, Washington, Utah, and Atlanta (23). Independent of racial and ethnic differences, patients with localized NSCLC in New Mexico had a greater risk of adjusted mortality (adjusted HR =1.22; 95% CI: 1.12–1.32) and lower rates of surgery compared to patients in other states (adjusted OR =0.46; 95% CI: 0.39–0.54; P<0.0001).
Osuoha et al. compared rates of resection and survival for lung cancer patients within the state of Nevada (25). These authors demonstrated that patients with localized NSCLC from rural and Southern Nevada were significantly more likely to not undergo surgery than patients from Northwestern Nevada with similarly staged disease (rural: OR =1.65, 95% CI: 1.07–2.53; southern region: OR =1.67, 95% CI: 1.20–2.13). After adjusting for sex, age, race, marital status, insurance, and income, such patients were also at increased risk of morality.
Sineshaw et al. evaluated state-level variation in the receipt of curative-intent surgery for stage I–II NSCLC using data sourced from over 110,000 patients across 38 different states in the North America Association of Central Cancer Registry (27). The authors showed that rates of resection varied considerably across the states examined, ranging from 52.2% in Wyoming to 77.2% in Utah. Additionally, after controlling for demographic, tumor-related factors, and the year of diagnosis, patients in 28 states were significantly less likely to undergo curative resection than patients in Massachusetts (selected as the reference because it had the lowest rate of uninsured residents among the states included in the study). Since their models also accounted for insurance type, area-level poverty, and urban-rural status, their work suggests that additional factors within the state of residence influence the surgical management of lung cancer.
Special patient cohorts
Traditionally, much of the literature on lung cancer disparities has used data sourced from state or national registries. One challenge in characterizing treatment disparities in these large, heterogeneous datasets is the potentially confounding effect of uncontrolled variables that may influence access to healthcare services. To limit some of these residual confounders, some investigators have studied treatment and survival disparities in special patient cohorts with more predictive patterns of care and/or balanced demographic characteristics (Table 3). Here we focus on US Veterans, clinical trial participants, young and elderly patients, and members of sexual minority populations.
Full table
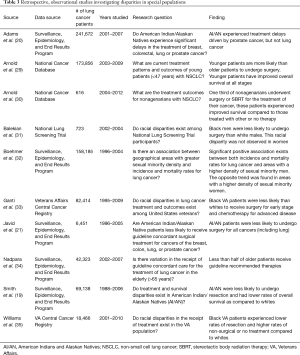
Ganti et al. evaluated the impact of race on surgical treatment in United States veterans with localized NSCLC from 1995–2009 (33). Despite having equal access to care through the Veterans Affairs (VA) Healthcare System, a significantly lower proportion of black patients than white patients underwent surgery for stage I disease (41% vs. 48%, P<0.001) and palliative chemotherapy for stage IV disease. Interestingly, even with lower rates of surgery, black patients did not demonstrate lower overall survival for stage I or II disease in this analysis.
Williams et al. published a follow up study on racial disparities in the VA population (35). Unlike the prior work by Ganti et al., Williams and colleagues provided data on the annual rates of resection and highlighted significantly different trends between black and white patients. Whereas the proportion of white patients undergoing resection did not vary from 2001 to 2010, the rate of resection among black patients increased by 7% during this period (49% in 2001 to 56% in 2010). When analyzing the entire study cohort, these authors found that black patients experienced lower rates of resection and higher rates of non-surgical or no treatment as compared to white patients, corroborating Ganti’s prior findings.
Balekian et al. assessed whether surgical disparities were present among stage I NSCLC patients previously enrolled in the National Lung Screening Trial (NLST) (31). In accordance with the NLST study criteria, patients were randomized to undergo routine lung cancer screening with low-dose CT or chest radiography. Given that the study cohort was enrolled in this clinical trial, the authors presumed that such patients would be surgically fit and therefore hypothesized that surgical disparities, if present, would be observed in older patients with greater comorbidities. While older age was found to be significantly associated with a reduced likelihood of undergoing surgery, the authors also observed racial disparities in the receipt of surgery between black and white men (65% black men vs. 93% white men). This trend persisted in the adjusted analysis with a random effects model to control for enrollment center variation (OR =0.15, 95% CI: 0.05–0.43; RR =0.72, 95% CI: 0.50–0.99). Racial disparities were not observed among women, as 90% of black women and 93% of white women underwent surgery.
Age-related disparities have previously been described in the lung cancer literature. While age is frequently included as a covariate in treatment prediction models, for the purpose of this review, we chose to focus on studies that specifically looked at treatment and outcome patterns for NSCLC patients at relative extremes of age.
Nadpara et al. described the treatment patterns and factors associated with the receipt of guideline concordant care in elderly SEER-Medicare patients with resectable NSCLC (34). Notably, the authors found that only 44.3% of the study cohort received stage-specific guideline recommended therapies. They found that the odds of receiving guideline concordant care were inversely correlated with age, as patients aged 66–69 were more than twice as likely to receive appropriate treatment than those ≥80 years (OR =2.66; 95% CI: 2.44–2.89) (34). Though this model controlled for patient comorbidity using modified Charlson comorbidity scores, other relevant factors such as pulmonary function, physiologic tolerance, and patient preference for surgery were not included.
Arnold et al. evaluated the effectiveness of local therapy for the treatment of stage I NSCLC in nonagenarians in the NCDB (30). These authors defined local therapy as the receipt of surgery or stereotactic body radiation therapy (SBRT). Among those who received local therapy, 37% underwent resection and 63% underwent SBRT. Patients that underwent local therapy had significantly improved overall 5-year survival compared to those with other therapy or no treatment (23% local vs. 13% other vs. 8% no therapy, P<0.0001) (30). The therapeutic benefit conferred by local surgical or SBRT therapy in this study suggests that old age alone should not be viewed as a contraindication to the use of such therapies.
On the other end of the spectrum, patients under the age of 55 currently represent approximately 8.6% of incident lung cancer cases in the country (36). In contrast to the average lung cancer patient, this younger cohort tends to be female, non-white, with fewer comorbidities and more advanced disease at the time of diagnosis (29,37-40). Studies in both SEER and the NCDB have independently shown that young lung cancer patients demonstrate significantly better stage-specific survival as compared to older patient cohorts (29,38-40). In one example, Arnold et al. evaluated stage-specific treatment patterns in the NCDB and found that a greater proportion of patients aged 20–46 with stage I–II NSCLC received surgery (± adjuvant chemotherapy) than older patients with equivalently staged disease (29). Moreover, after controlling for other clinical and tumor related variables, including comorbidity status, the authors found that older stage II patients remained more likely to receive radiation therapy only, which was associated with an increased risk of mortality in their model (HR =2.32, P<0.0001) (29). While this analysis demonstrated that young lung cancer patients are more likely than older patients to receive appropriate treatment for early and advanced NSCLC, it did not investigate the factors associated with treatment or survival within the younger cohort.
Sexual minority populations (lesbian, gay, and bisexual), reflect an emerging patient cohort in which disproportionate rates of cancer incidence and mortality have been reported (41-43). In a SEER analysis using same-sex patient households as a surrogate for sexual minority status, Boehmer et al. observed a significant association between sexual minority density and lung cancer incidence and mortality (32). After correcting for race, education, and poverty, the authors found lung cancer incidence and mortality to be positively correlated with male sexual minority density [incidence rate ratio (IRR) =1.05, 95% CI: 1.04–1.07, P<0.0001; mortality rate ratio (MRR) =1.03, 95% CI: 1.01–1.05, P=0.0083] (32). The impact of sexual minority status on the receipt of lung cancer surgery has not been investigated, but these studies suggest that additional focus on surgical treatments and outcome patterns in sexual minority populations with lung cancer is warranted.
Potential mechanisms that perpetuate underlying surgical disparities for the treatment of early stage NSCLC
Though significant progress has been made in the identification of NSCLC patient groups at risk of not receiving guideline-recommended surgery, the causal determinants of these discrepancies remain unclear. Studies in the literature have shown that differences in patient, physician, and health system factors may, at least in part, perpetuate the ongoing trends in surgical disparities reported in lung cancer patients.
Patient-mediated mechanisms
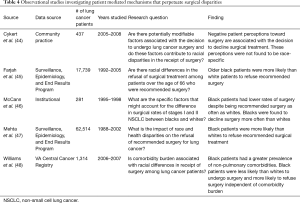
One potential mechanism underlying differences in the receipt of surgery is patient preference for surgery and treatment in general (Table 4). Multiple studies have reported a disproportionate rate of refusal to undergo surgery among black patients with early stage NSCLC. In an institutional series of 281 patients, McCann et al. described a significantly lower rate of surgery among black patients despite no significant differences in the recommendation to undergo surgery (black 70% vs. white 79%, P=0.11) (46). On further evaluation, it was found that black patients were disproportionately more likely to decline surgery than white patients (18% vs. 5%, P=0.002) (46). Higher rates of refusal of surgery have also been reported in the SEER population. In a cohort of stage I–II NSCLC patients over the age of 50, Mehta et al. found that after adjusting for age, sex, and marital status, black patients and patients of other race were at increased odds of refusing surgical treatment as compared to white patients (black: OR =1.88, 95% CI: 1.50–2.36, P<0.001; other: OR =1.95, 95% CI: 1.50–2.52, P<0.001) (47). An earlier study by Farjah et al. investigated whether racial differences exist among SEER-Medicare patients over the age of 66 who were recommended surgery for stage I–II disease (45). They reported a 14% difference in the proportion of black and white patients that underwent resection (black 69% vs. white 83%). Though this study did not specifically mention why patients did not undergo resection, variation in patient preference cannot be ruled out.
Full table
Williams et al. investigated racial differences in attitudes toward surgery in a cohort of VA patients (48). After stratifying patients by comorbidity burden, they showed that a greater proportion of black patients did not receive surgery in each comorbidity category (mild, moderate, severe) as compared to white patients. In addition, black patients were also more likely than whites to refuse surgery in each severity category. Like Farjah et al., these authors did not include data on the reasons for patient refusal of surgery (45).
Cykert et al. examined the factors associated with the decision to undergo surgery among patients with newly diagnosed stage I–II NSCLC in North and South Carolina (44). Similar to Williams et al., they described lower rates of surgery among black patients with high morbidity burden (OR =0.04; 95% CI: 0.01–0.25) not observed in highly comorbid white patients (OR =0.45; 95% CI: 0.10–2.0) (48). Additionally, the authors identified multiple potentially modifiable factors associated with the decision to not undergo surgery, including the patient’s belief that the lung cancer diagnosis was less than 90% certain (OR =0.37; 95% CI: 0.14–0.93), negative perceptions of patient-physician communication (OR =0.42; 95% CI: 0.32–0.74) and negative perceptions of prognosis 1 year following surgery (OR =0.27; 95% CI: 0.14–0.50) (44). Though these beliefs were not found to be race-specific, other reports have described racial differences in perceptions toward surgery and physician communication (49,50).
Disparities in staging evaluation
NCCN guidelines recommend timely and appropriate staging and surgical evaluation following the diagnosis of lung cancer (5). In keeping with these recommendations, the use of positron emission tomography (PET) imaging and other mediastinal staging modalities have been shown to improve staging accuracy and prevent unnecessary surgery in NSCLC patients (51-55). However, significant underutilization and variability in the use of these advanced staging techniques has been reported (Table 5) (56-58,60-62).

Full table
Gould et al. conducted a prospective cohort analysis of disparities in PET staging for 3,638 patients with newly diagnosed stage I–II NSCLC from 2003 to 2005 enrolled in the Cancer Care Outcomes Research and Surveillance Consortium (57). This consortium includes patients from four different geographical regions, representing approximately 10% of the United States population. The authors found PET use to be significantly lower among patients who were older than 69 years of age (RR =0.91; 95% CI: 0.81–1.00), of non-white race (RR =0.87; 95% CI: 0.77–0.97), had Medicare insurance (RR =0.87; 95% CI: 0.76–0.99), or had less than 9 years of formal schooling (RR =0.76; 95% CI: 0.57–0.98) (57).
Lathan et al. examined the impact of race on patterns of invasive staging and treatment in SEER-Medicare patients (58). These authors found black race to be independently associated with reduced odds of undergoing invasive staging (OR =0.75; 95% CI: 0.67–0.83) and surgery (OR =0.55; 95% CI: 0.47–0.64) (58). When evaluating the potential reasons for this discrepancy, they found that black patients were recommended surgery less often (67% vs. 71.4%, P<0.05) and had higher rates of refusal for surgery (3.4% vs. 2.0%, P<0.05) than white patients (58).
Farjah et al. assessed the patterns and factors associated with the use of single (CT), bi- (CT + PET or CT + invasive staging), and tri-modality (CT + PET + invasive staging) staging in SEER-Medicare beneficiaries with NSCLC from 1998 to 2005 (56). These authors found that between 1998 and 2002, there was a statistically significant decrease the use of single modality staging (90% to 67%, P<0.001) and a reciprocal increase in the use of multi-modality staging techniques (bi-modality 10% to 30%; tri-modality 0.4% to 5%) (56). The increase in multi-modal staging appeared to be driven primarily by increases in PET (2% to 31%, P<0.001) as compared to invasive techniques, which decreased over the study period (9% to 8%, P=0.005) (56). The authors identified several demographic and socioeconomic status factors associated with lower odds of undergoing multi-modal staging by multivariable regression, including increased age (OR =0.97; 95% CI: 0.96–0.97), black race (OR =0.63; 95% CI: 0.55–0.72), low income (OR =0.84; 95% CI: 0.77–0.93), low education (OR =0.89; 95% CI: 0.81–0.97), living in the Mid-West (OR 0.68, 95% CI: 0.62–0.74), and rural area of residence (OR =0.80; 95% CI: 0.71–0.91) (56). In contrast, Suga et al. reported no association between race and non-invasive or invasive staging in a cohort of patients from the California Cancer Registry (59). Instead, these authors found that patients residing in rural areas were less likely to undergo non-invasive staging (PET, MRI, and CT) as well as invasive staging modalities (bronchoscopy, mediastinoscopy, and thoracoscopy) (59). These data suggest that the factors underlying staging discrepancies may differ according to region.
Collectively, these studies outline significant disparities in the receipt of staging. Given the importance of timely and accurate staging in the evaluation of surgical candidacy, staging disparities may subsequently perpetuate treatment disparities.
Geographic/travel distance
The literature concerning the impact of travel distance on resource utilization and clinical outcomes for cancer surgery has been mixed (Table 6). In some studies, increased travel distance has been shown to be associated with later stage at diagnosis, longer time to treatment initiation, and lower rates of surgery (65-69). Conversely, other studies have found that cancer patients who travel greater distances are more likely to receive care at higher volume centers, undergo surgical resection, and experience improved outcomes (70-72). Unfortunately, data specifically evaluating the relationship between travel distance and surgical outcomes in the United States lung cancer population are limited.

Full table
In an observational study of lung cancer patients undergoing resection in the state of New York, Lieberman-Cribben et al. found that patients living further away from high volume or very high volume centers (top two quintiles of surgical volume) had significantly lower odds of undergoing surgery at such centers (>2.3–6.1 miles: OR =0.51, 95% CI: 0.46–0.56; >6.1 miles: OR =0.27, 95% CI: 0.24–0.30) and were concomitantly more likely to undergo surgery at low volume or very low volume centers (>2.3–6.1 miles: OR =1.44, 95% CI: 1.23–1.67; >6.1 miles: OR =2.70, 95% CI: 2.34–3.13) (63). This trend was maintained in a subgroup analysis comparing white and black patients.
In a second study published by the same group, the authors assessed the factors associated with the receipt of lobectomy (64). In this analysis, non-Hispanic ethnicity (OR =2.25; 95% CI: 1.60–3.16), other race/ethnicity (OR =1.41; 95% CI: 1.16–1.71), greater Elixhauser comorbidity index (OR =1.02; 95% CI: 1.01–1.02), and greater median income (OR =2.88; 95% CI: 2.51–3.32) were each found to be independently associated with a greater likelihood of undergoing lobectomy at a distant high volume hospital (64). In contrast, black race (OR =1.69; 95% CI: 1.26–2.26) and Hispanic ethnicity (OR =2.27; 95% CI: 1.51–3.42) were associated with increased odds of undergoing lobectomy at a distant low volume hospital (64). Importantly, patients treated at distant low volume centers were more likely to experience adverse postoperative events compared to those treated at near high volume centers (OR =1.49; 95% CI: 1.25–1.78). Together these data suggest that racial/ethnic differences in travel patterns may profoundly affect the quality of care received for the treatment of NSCLC, though additional research into the impact of travel distance and the receipt of surgery in nationally representative cohorts is necessary.
Stigma
In recent years, there has been a growing public health awareness and concern surrounding the negative stigma associated with a lung cancer diagnosis and its potential impact on patient care and outcomes. Lung cancer patients have been shown to experience high rates of perceived and internalized negative stigma because of the association with smoking secondary to anti-tobacco advertising campaigns, along with depressive and other psychological symptoms (Table 7) (74-76,78,79). Additionally, rates of self-reported negative stigmas and shame were found to be higher among lung cancer patients than patients with breast or prostate cancer (76). Within the lung cancer population, negative self-perception has been shown to differ based on factors such as smoking status, with current/former smokers reporting higher levels of internalized stigma, personal responsibility, and regret as compared to never smokers (74,79).
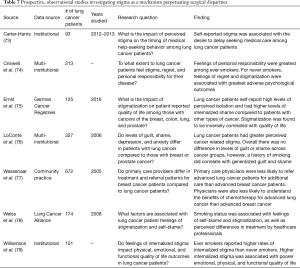
Full table
In addition to its deleterious effects on overall quality of life, patient and physician perceptions of stigma about lung cancer may negatively impact patient care. Wassenaar et al. conducted a study in which 672 primary care physicians were surveyed using clinical scenarios designed to assess treatment and referral patterns for hypothetical breast cancer and lung cancer patients (77). The authors found that primary care physician respondents were less likely to refer advanced lung cancer patients to an oncologist than advanced breast cancer patients, irrespective of performance status. Respondents also believed that chemotherapy was less effective for lung cancer patients than for stage-matched breast cancer patients. Remarkably, these hypothetical survey answers were given despite the two groups of primary care physicians treating similar proportions of breast and lung cancer patients in their actual clinical practice.
As for the implication of perceived stigma on patient attitudes, Carter-Harris et al. published a study showing an association between increased patient reported negative stigma and the desire to delay receiving medical care (73). Ultimately, no study to date has reported disparities in the receipt of surgery for NSCLC based solely on perceptions such as stigma and nihilism. However, given the potential additive effect of these perceptions across the patient, physician, and healthcare system levels, an assessment of the potential impact of these factors on the surgical and clinical care of NSCLC patients may be warranted (80).
Discussion
Surgical resection remains a fundamental component of the therapeutic management of NSCLC. For patients with localized disease, curative intent surgery offers the best clinical and oncologic outcomes. While it is currently estimated that 30% of NSCLC patients have resec disease on clinical evaluation, this number is expected to increase with recent national guidelines endorsing annual low-dose computed tomography screening in high risk patients (5,81-83). Conversely, in patients with advanced disease, surgery has conventionally occupied a diagnostic or palliative role. However, recent evidence suggests that multimodal treatment regimens that include surgical resection may confer a therapeutic benefit in select patients with metastatic NSCLC (84). Given the expanding indications and utility for thoracic surgery in the management of NSCLC of all stages, efforts to identify and mitigate disparities in the use of surgery have become exceedingly relevant from a clinical and public health perspective.
In this review, we summarize nearly two decades of surgical disparities literature and highlight common and unrecognized disparities in the receipt of surgery. We demonstrate that inequity persists in the management of lung cancer patients, with racial, socioeconomic, and other special patient groups are at risk of not receiving guideline recommended surgery. While improving effective communication and access to staging and other resources are important areas to target, further study into the mechanisms perpetuating these disparities are warranted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Key Statistics for Lung Cancer. Vol. 5, American Cancer Society, 2017:1-3. Available online: https://www.cancer.org/cancer/lung-cancer.html
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Houston KA, Mitchell KA, King J, et al. Histologic Lung Cancer Incidence Rates and Trends Vary by Race/Ethnicity and Residential County. J Thorac Oncol 2018;13:497-509. [Crossref] [PubMed]
- Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: A national cancer database survey. J Thorac Oncol 2010;5:29-33. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013;123:188S-201S.
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341:1198-205. [Crossref] [PubMed]
- Chang CM, Su YC, Lai NS, et al. The Combined Effect of Individual and Neighborhood Socioeconomic Status on Cancer Survival Rates. Ganti AK, editor. PLoS One 2012;7:e44325.
- Yang LL, Zhang XC, Yang XN, et al. Lung Cancer Treatment Disparities in China: A Question in Need of an Answer. Oncologist 2014;19:1084-90. [Crossref] [PubMed]
- Berglund A, Holmberg L, Tishelman C, et al. Social inequalities in non-small cell lung cancer management and survival: a population-based study in central Sweden. Thorax 2010;65:327-33. [Crossref] [PubMed]
- Cartman ML, Hatfield AC, Muers MF, et al. Lung cancer: district active treatment rates affect survival. J Epidemiol Community Health 2002;56:424-9. [Crossref] [PubMed]
- McMahon M, Barbiere JM, Greenberg DC, et al. Population-based trends in use of surgery for non-small cell lung cancer in a UK region, 1995-2006. Thorax 2011;66:453-5. [Crossref] [PubMed]
- Wisnivesky JP, McGinn T, Henschke C, et al. Ethnic disparities in the treatment of stage I non-small cell lung cancer. Am J Respir Crit Care Med 2005;171:1158-63. [Crossref] [PubMed]
- Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of Surgical Resection for Localized, Non-Small Cell Lung Cancer Among Whites and African Americans in South Carolina. Ann Thorac Surg 2008;86:220-6; discussion 227. [Crossref] [PubMed]
- Hardy D, Xia R, Liu CC, et al. Racial disparities and survival for nonsmall-cell lung cancer in a large cohort of black and white elderly patients. Cancer 2009;115:4807-18. [Crossref] [PubMed]
- Soneji S, Tanner NT, Silvestri GA, et al. Racial and Ethnic Disparities in Early-Stage Lung Cancer Survival. Chest 2017;152:587-97. [Crossref] [PubMed]
- Hamid MS, Shameem R, Gafoor K, et al. Non-small-cell lung cancer clinicopathologic features and survival outcomes in Asian pacific islanders residing in the united states: A SEER analysis. J Cancer Epidemiol 2015;2015:269304. [Crossref] [PubMed]
- Saeed AM, Toonkel R, Glassberg MK, et al. The influence of Hispanic ethnicity on nonsmall cell lung cancer histology and patient survival: An analysis of the Survival, Epidemiology, and End Results database. Cancer 2012;118:4495-501. [Crossref] [PubMed]
- Smith CB, Bonomi M, Packer S, et al. Disparities in lung cancer stage, treatment and survival among American Indians and Alaskan Natives. Lung Cancer 2011;72:160-4. [Crossref] [PubMed]
- Adams SV, Bansal A, Burnett-Hartman AN, et al. Cancer Treatment Delays in American Indians and Alaska Natives Enrolled in Medicare. J Health Care Poor Underserved 2017;28:350-61. [Crossref] [PubMed]
- Javid SH, Varghese TK, Morris AM, et al. Guideline-concordant cancer care and survival among American Indian/Alaskan Native patients. Cancer 2014;120:2183-90. [Crossref] [PubMed]
- Atkins GT, Kim T, Munson J. Residence in rural areas of the United States and lung cancer mortality: Disease incidence, treatment disparities, and stage-specific survival. Ann Am Thorac Soc 2017;14:403-11. [Crossref] [PubMed]
- Crowell RE, Goetz T, Wiggins C, et al. Regional Disparities in Treatment and Survival of Early Stage Non-Small Cell Lung Cancer. Ethn Dis 2007;17:358-64. [PubMed]
- Johnson AM, Johnson A, Hines RB, et al. The effects of residential segregation and neighborhood characteristics on surgery and survival in patients with early-stage non?small cell lung cancer. Cancer Epidemiol Biomarkers Prev 2016;25:750-8. [Crossref] [PubMed]
- Osuoha CA, Callahan KE, Ponce CP, et al. Disparities in lung cancer survival and receipt of surgical treatment. Lung Cancer 2018;122:54-9. [Crossref] [PubMed]
- Shi R, Diaz R, Shi Z, et al. The Effect of Payer Status on Survival of Patients with Stage I/II Non-small Cell Lung Cancer: NCDB 1998-2011. Anticancer Res 2016;36:319-26. [PubMed]
- Sineshaw HM, Wu XC, Dana Flanders W, et al. Variations in receipt of curative-intent surgery for early-stage non-small cell lung cancer (NSCLC) by state. J Thorac Oncol 2016;11:880-9. [Crossref] [PubMed]
- Yorio JT, Yan J, Xie Y, et al. Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center. Clin Lung Cancer 2012;13:448-57. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Rosen JE, et al. Lung cancer in the very young: Treatment and survival in the national cancer data base. J Thorac Oncol 2016;11:1121-31. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Rosen JE, et al. Effectiveness of local therapy for stage I non-small-cell lung cancer in nonagenarians. Surgery 2017;162:640-51. [Crossref] [PubMed]
- Balekian AA, Wisnivesky JP, Gould MK. Surgical Disparities Among Patients With Stage I Lung Cancer in the National Lung Screening Trial. Chest 2019;155:44-52. [Crossref] [PubMed]
- Boehmer U, Ozonoff A, Miao X. An ecological approach to examine lung cancer disparities due to sexual orientation. Public Health 2012;126:605-12. [Crossref] [PubMed]
- Ganti AK, Subbiah SP, Kessinger A, et al. Association between race and survival of patients with non-small-cell lung cancer in the united states veterans affairs population. Clin Lung Cancer 2014;15:152-8. [Crossref] [PubMed]
- Nadpara PA, Madhavan SS, Tworek C, et al. Guideline-concordant lung cancer care and associated health outcomes among elderly patients in the United States. J Geriatr Oncol 2015;6:101-10. [Crossref] [PubMed]
- Williams CD, Salama JK, Moghanaki D, et al. Impact of Race on Treatment and Survival among U.S. Veterans with Early-Stage Lung Cancer. J Thorac Oncol 2016;11:1672-81. [Crossref] [PubMed]
- Surveillance, epidemiology, and end results program, Statistical summaries. Cancer stat fact sheets, Cancer of the lung and bronchus. Available online: https://seer.cancer.gov/statfacts/html/lungb.html
- Skarin AT, Herbst RS, Leong TL, et al. Lung cancer in patients under age 40. Lung Cancer 2001;32:255-64. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: A Surveillance, Epidemiology, and End Results (SEER) analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Thomas A, Chen Y, Yu T, et al. Trends and Characteristics of Young Non-Small Cell Lung Cancer Patients in the United States. Front Oncol 2015;5:113. [Crossref] [PubMed]
- Jemal A, Miller KD, Ma J, et al. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N Engl J Med 2018;378:1999-2009. [Crossref] [PubMed]
- Boehmer U, Ozonoff A, Miao X. An ecological analysis of colorectal cancer incidence and mortality: Differences by sexual orientation. BMC Cancer 2011;11:400. [Crossref] [PubMed]
- Boehmer U, Ozonoff A, Timm A. County-level association of sexual minority density with breast cancer incidence: Results from an ecological study. Sex Res Soc Policy 2011;8:139-45. [Crossref]
- Boehmer U, Miao X, Maxwell NI, et al. Sexual minority population density and incidence of lung, colorectal and female breast cancer in California. BMJ Open 2014;4:e004461. [Crossref] [PubMed]
- Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA 2010;303:2368-76. [Crossref] [PubMed]
- Farjah F, Wood DE, Yanez ND, et al. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg 2009;144:14-8. [Crossref] [PubMed]
- McCann J, Artinian V, Duhaime L, et al. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest 2005;128:3440-6. [Crossref] [PubMed]
- Mehta RS, Lenzner D, Argiris A. Race and health disparities in patient refusal of surgery for early-stage non-small cell lung cancer: A SEER cohort study. Ann Surg Oncol 2012;19:722-7. [Crossref] [PubMed]
- Williams CD, Stechuchak KM, Zullig LL, et al. Influence of comorbidity on racial differences in receipt of surgery among US veterans with early-stage non-small-cell lung cancer. J Clin Oncol 2013;31:475-81. [Crossref] [PubMed]
- Margolis ML, Christie JD, Silvestri GA, et al. Racial Differences Pertaining to a Belief about Lung Cancer Surgery. Ann Intern Med 2003;139:558-63. [Crossref] [PubMed]
- Gordon HS, Street RL, Sharf BF, et al. Racial differences in trust and lung cancer patients’ perceptions of physician communication. J Clin Oncol 2006;24:904-9. [Crossref] [PubMed]
- Pieterman RM, Van Putten JW, Meuzelaar JJ, et al. Preoperative Staging of Non-Small Cell Lung Cancer with Positron-Emission Tomography. N Engl J Med 2000;343:254-61. [Crossref] [PubMed]
- van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: The PLUS multicentre randomised trial. Lancet 2002;359:1388-93. [Crossref] [PubMed]
- Gonzalez-Stawinski GV, Lemaire A, Merchant F, et al. A comparative analysis of positron emission tomography and mediastinoscopy in staging non-small cell lung cancer. J Thorac Cardiovasc Surg 2003;126:1900-5. [Crossref] [PubMed]
- Reed CE, Harpole DH, Posther KE, et al. Results of the American College of Surgeons Oncology Group Z0050 Trial: The utility of positron emission tomography in staging potentially operable non-small cell lung cancer. J Thorac Cardiovasc Surg 2003;126:1943-51. [Crossref] [PubMed]
- Gao SJ, Kim AW, Puchalski JT, et al. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer 2017;109:36-41. [Crossref] [PubMed]
- Farjah F, David RF, Scott DR, et al. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol 2009;4:355-63. [Crossref] [PubMed]
- Gould MK, Schultz EM, Wagner TH, et al. Disparities in lung cancer staging with positron emission tomography in the cancer care outcomes research and surveillance (cancors) study. J Thorac Oncol 2011;6:875-83. [Crossref] [PubMed]
- Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol 2006;24:413-8. [Crossref] [PubMed]
- Suga JM, Nguyen D V., Mohammed SM, et al. Racial disparities on the use of invasive and noninvasive staging in patients with non-small cell lung cancer. J Thorac Oncol 2010;5:1772-8. [Crossref] [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6. [Crossref] [PubMed]
- Thornblade LW, Wood DE, Mulligan MS, et al. Variability in invasive mediastinal staging for lung cancer: A multicenter regional study. J Thorac Cardiovasc Surg 2018;155:2658-2671.e1. [Crossref] [PubMed]
- Krantz SB, Howington JA, Wood DE, et al. Invasive Mediastinal Staging for Lung Cancer by The Society of Thoracic Surgeons Database Participants. Ann Thorac Surg 2018;106:1055-62. [Crossref] [PubMed]
- Lieberman-Cribbin W, Liu B, Leoncini E, et al. Temporal trends in centralization and racial disparities in utilization of high-volume hospitals for lung cancer surgery. Medicine (Baltimore) 2017;96:e6573. [Crossref] [PubMed]
- Liu B, Flores RM, Taioli E. Patterns of elective lobectomy for lung cancer. J Surg Res 2017;220:59-67. [Crossref] [PubMed]
- Nattinger AB, Ronald T, Hoffmann RG, et al. Relationship of Distance From a Breast Cancer Treatment. J Natl Cancer Inst 2001;93:1344-6. [Crossref] [PubMed]
- Jones AP, Haynes R, Sauerzapf V, et al. Travel time to hospital and treatment for breast, colon, rectum, lung, ovary and prostate cancer. Eur J Cancer 2008;44:992-9. [Crossref] [PubMed]
- Scoggins JF, Fedorenko CR, Donahue SMA, et al. Is Distance to provider a barrier to care for medicaid patients with breast, colorectal, or lung cancer? J Rural Health 2012;28:54-62. [Crossref] [PubMed]
- Tracey E, Mccaughan B, Badgery-Parker T, et al. Patients with localized non-small cell lung cancer miss out on curative surgery with distance from specialist care. ANZ J Surg 2015;85:658-63. [Crossref] [PubMed]
- Tracey E, McCaughan B, Badgery-Parker T, et al. Distance from accessible specialist care and other determinants of advanced or unknown stage at diagnosis of people with non-small cell lung cancer: A data linkage study. Lung Cancer 2015;90:15-21. [Crossref] [PubMed]
- Wasif N, Chang YH, Pockaj BA, et al. Association of Distance Traveled for Surgery with Short- and Long-Term Cancer Outcomes. Ann Surg Oncol 2016;23:3444-52. [Crossref] [PubMed]
- Vetterlein MW, Löppenberg B, Karabon P, et al. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer 2017;123:3241-52. [Crossref] [PubMed]
- Graboyes EM, Ellis MA, Li H, et al. Racial and Ethnic Disparities in Travel for Head and Neck Cancer Treatment and the Impact of Travel Distance on Survival. Cancer 2018;124:3181-91. [Crossref] [PubMed]
- Carter-Harris L. Lung cancer stigma as a barrier to medical help-seeking behavior: Practice implications. J Am Assoc Nurse Pract 2015;27:240-5. [Crossref] [PubMed]
- Criswell KR, Owen JE, Thornton AA, et al. Personal responsibility, regret, and medical stigma among individuals living with lung cancer. J Behav Med 2016;39:241-53. [Crossref] [PubMed]
- Ernst J, Mehnert A, Dietz A, et al. Perceived stigmatization and its impact on quality of life - results from a large register-based study including breast, colon, prostate and lung cancer patients. BMC Cancer 2017;17:741-8. [Crossref] [PubMed]
- LoConte NK, Else-Quest NM, Eickhoff J, et al. Assessment of guilt and shame in patients with non-small-cell lung cancer compared with patients with breast and prostate cancer. Clin Lung Cancer 2008;9:171-8. [Crossref] [PubMed]
- Wassenaar TR, Eickhoff JC, Jarzemsky DR, et al. Differences in primary care clinicians’ approach to non-small cell lung cancer patients compared with breast cancer. J Thorac Oncol 2007;2:722-8. [Crossref] [PubMed]
- Weiss J, Yang H, Weiss S, et al. Stigma, self-blame, and satisfaction with care among patients with lung cancer. J Psychosoc Oncol 2017;35:166-79. [Crossref] [PubMed]
- Williamson TJ, Choi AK, Kim JC, et al. A Longitudinal Investigation of Internalized Stigma, Constrained Disclosure, and Quality of Life Across 12 Weeks in Lung Cancer Patients on Active Oncologic Treatment. J Thorac Oncol 2018;13:1284-93. [Crossref] [PubMed]
- Hamann HA, Ver Hoeve ES, Carter-Harris L, et al. Multilevel Opportunities to Address Lung Cancer Stigma across the Cancer Control Continuum. J Thorac Oncol 2018;13:1062-75. [Crossref] [PubMed]
- Moyer VA. U.S. Preventive Services Task Force. Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2014;160:330-8. [PubMed]
- Wender R, Fontham ET, Barrera E Jr, et al. American Cancer Society Lung Cancer Screening Guidelines. CA Cancer J Clin 2013;63:107-17. [Crossref] [PubMed]
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. [Crossref] [PubMed]
- David EA, Clark JM, Cooke DT, et al. The Role of Thoracic Surgery in the Therapeutic Management of Metastatic Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1636-45. [Crossref] [PubMed]


