
Interpretation of venous-to-arterial carbon dioxide difference in the resuscitation of septic shock patients
Introduction
The evaluation and correction of macrocriculatory and microcirculatory flow play an important role in the resuscitation of circulatory shock (1). The venous-to-arterial carbon dioxide difference [P(v-a)CO2] has gained great attentions in the resuscitation of sepsis. The P(v-a)CO2 is determined by cardiac output and metabolic status, and it has been taken as an indicator of the adequacy of the venous blood flow to remove the CO2 produced by the peripheral tissues (2,3).
The P(v-a)CO2 was calculated as the difference between venous PCO2 and arterial PCO2. The venous PCO2 could be obtained from the mixed venous blood through a pulmonary artery catheter or from the central venous blood through a central venous catheter. Researches (4,5) had shown that central venous-arterial PCO2 difference [P(cv-a)CO2] was consistent with mixed venous-arterial PCO2 difference [P(mv-a)CO2] and both of them were inversely related to cardiac index (CI). Nowadays, the central venous PCO2 is commonly used to calculate P(v-a)CO2 in clinical practice. Recent study found that P(mv-a)CO2 might be a potential indicator to reflect microcirculatory flow in septic shock patients (6). In this paper, we review the literatures of P(v-a)CO2 and try to answer the question how to interpret and manage the P(v-a)CO2 in the resuscitation of sepsis.
P(v-a)CO2 and prognosis in sepsis
Based on the physiological background of P(v-a)CO2, it is easy to understand that a high P(v-a)CO2 indicate an impaired cardiac output and tissue hypoperfusion. Hence, a persistent P(v-a)CO2 after resuscitation is related to a poor prognosis in septic shock patients (6). A cutoff 6 mmHg of P(v-a)CO2 has been suggested as an indicator to reflect the adequacy of cardiac output to tissue perfusion in critically ill patients (3). Several studies had reported that a high P(v-a)CO2 (>6 mmHg) was related to poor outcome in septic shock condition (4,7-12). van Beest et al. (4) found that a high P(cv-a)CO2 (≥6 mmHg) in the first 24 h after ICU admission was related to a higher hospital mortality rate (OR 5.3, P=0.08) in 53 septic shock patients. Vallee et al. (7) further reported that the septic shock patients with a higher P(cv-a)CO2 had a poor lactate clearance, higher SOFA score, and a lower mortality rate, in the normalized central venous oxygen saturation (ScvO2) (>70%) condition, than patients with a normal P(cv-a)CO2 value (<6 mmHg). Moreover, Mallat et al. (8) reported that P(cv-a)CO2 was not related to 28-day mortality in septic shock patients. But the authors found that normalization of both P(cv-a)CO2 gap and ScvO2, during the first 6 hours of resuscitation, was associated with a better lactate clearance than the normalization of ScvO2 alone (8). Therefore, P(cv-a)CO2 was suggested as an additional goal of resuscitation when ScvO2 target had been achieved (>70%) in septic shock patients (7,8).
Moreover, our study found a lower P(cv-a)CO2 (3.5 mmHg but not 6 mmHg) had a good ability for predicting ICU mortality in septic shock patients with a high ScvO2 (>80%) (13). The non-survivor group had a low P(v-a)CO2 (mean 4.8 mmHg) <6 mmHg and high lactate level (mean 3.1 mmol/L) in our study. Hence, the normal cutoff value of P(v-a)CO2 requires further investigations to be validated in septic shock patients with a high ScvO2 (>80%) and signs of tissue hypoxia.
Recently, a systematic review showed that P(v-a)CO2 was correlated with mortality and other clinical outcomes in septic shock patients (14). Furthermore, Muller et al. (12) found that P(cv-a)CO2 was only associated with mortality in patients with impaired cardiac function (defined as atrial fibrillation and/or left ventricular ejection fraction less than 50%) but not with patients with normal cardiac function. The authors found that patients with septic shock and impaired cardiac function were more prone to a persistent high P(cv-a)CO2, even when initial resuscitation succeeded in normalizing mean arterial pressure, central venous pressure, and ScvO2 (12). In other words, a high P(cv-a)CO2 might mainly result from a poor cardiac function in the resuscitation of septic shock patients. Further clinical investigation is required to clarify the predictive meaning of P(cv-a)CO2 in normal cardiac function. The relevant clinical studies of P(cv-a)CO2 and outcome were summarized in the Table 1.

Full table
Pitfalls of P(v-a)CO2 in assessing global flow and tissue perfusion
There were some potential pitfalls of using P(v-a)CO2 to identify global flow and tissue perfusion in clinical situations.
- Hyperoxia: Saludes et al. (15) found that an elevated P(v-a)CO2 could independently result from a hyperoxia (caused by breathing 100% O2 for 5 min) but not from an inadequate cardiac output in the septic patients. Several potential mechanisms should be taken on how hyperoxia cause an increase in P(v-a)CO2 are as following: firstly, a high P(v-a)CO2 could be derived from the impaired microcirculatory flow caused by arterial hyperoxia (16). It has been shown that normobaric hyperoxia decreases capillary perfusion and VO2 and increases the heterogeneity of the perfusion (17). Secondly, Haldane effect, a phenomenon known as the increase in venous oxygen saturation would cause a decrease in the affinity of hemoglobin (Hb) for CO2 (18). The CO2 would unbind from Hb and, in the venous hyperoxia condition, would further produce an increase in the free form of CO2 in the venous site. Consequently, the P(v-a)CO2 would elevate in the high venous saturation condition resulted from hyperoxia (19).
- Hyper-ventilation: Mallat et al. (20) investigated the effect of acute hyperventilation on P(cv-a)CO2 gap in hemodynamically stable septic shock patients. The authors found that acute hyperventilation could increase P(cv-a)CO2 gap, which may be a result of increases in VO2. In other words, the acute changes in respiratory status could contribute to a high P(v-a)CO2, which might be independent of the changes in cardiac output. (III) Hypoxia: the cellular hypoxia could be caused by ischemic or hypoxic hypoxia. Vallet et al. found that P(v-a)CO2 increase in ischemic hypoxia induced by a decrease in blood flow, but not in hypoxic hypoxia conditions where the blood flow was maintained constant, even in a state of VO2/DO2 dependency, in a canine model of isolated limb (21). Hence, P(v-a)CO2 could serve as a marker of the adequacy of venous blood flow to wash-out the CO2 produced by the tissues (tissue hypoperfusion marker) rather than a marker of tissue hypoxia.
P(v-a)CO2 and microcirculation
Both ScvO2 and lactate have been well accepted as targets to guide resuscitation in sepsis (22). However, sometimes there might be some limitations in using ScvO2 and lactate to reflect tissue perfusion (23). For example, when capillary shunting occurred, ScvO2 could be elevated and mask the presence of tissue hypoperfusion or tissue hypoxia. Recently, P(v-a)CO2 has gained attention as a complementary tool to reflect global perfusion in the resuscitation of septic shock patients when ScvO2 is more than 70% (24).
Ospina-Tascon et al. (25) conducted a prospective study involving 75 septic shock patients with the aim to investigate the relationship between P(mv-a)CO2 and sublingual microcirculation assessed by sidestream dark-field device. They found that high P(mv-a)CO2 values were associated with low percentages of small perfused vessels (PPV), low functional capillary density, and high heterogeneity of microvascular blood flow. Interestingly, the relationship between P(v-a)CO2 and microcirculation was independent of the effects of cardiac output in that study. In summary, a high P(v-a)CO2 might be caused by an inadequate microcirculatory flow to clear the excess CO2 production, even in the presence of normal (or high) cardiac output in septic shock patients. Moreover, Kanoore et al. (26) found sepsis patients with a high CI (>4 L/min/m2) showed a lower P(v-a)CO2 (5±3 vs. 7±2 mmHg) than those with normal cardiac output. However, there were no differences in sublingual perfused vascular density, proportion of perfused vessels, or microvascular flow index in both groups in that study. Hence, an impaired microcirculation could be persistent even in a low P(v-a)CO2 and a high cardiac output condition. The loss of coherence between macrocirculation and microcirculation is common in septic shock patients (27). Importantly, it is uncertain if the decrease in P(v-a)CO2, observed after an increase in cardiac output, is related to the improvement of microcirculation. Further studies are needed to investigate this issue.
How to Interpret and manage a high P(v-a)CO2 (>6 mmHg)
An elevated P(v-a)CO2 could result from different reasons in septic shock patients, such as low cardiac output, poor microcirculatory perfusion or acute hyperventilation (28). Hence, a high P(v-a)CO2 should be taken as an alarm trigger of inadequate blood flow in the resuscitation of septic shock patients. It remains a challenge for intensivists to correctly interpret and manage an elevated P(v-a)CO2 (>6 mmHg) condition. In Figure 1, we summarized a recursive and regression approach of resuscitation of septic shock patients. The usefulness of this resuscitation protocol needs to be validated in clinical trials.
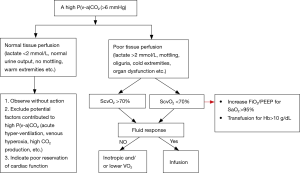
Conclusions
During recent years, P(v-a)CO2 has gained great attention and more frequently used in the resuscitation of septic shock patients. The intensivists should take other tissue perfusion parameters into consideration before correcting an elevated P(v-a)CO2 in the resuscitation of septic shock patients. Moreover, further investigations are necessary to clarify the relationship between P(v-a)CO2 and microcirculation.
Acknowledgments
Funding: This work was supported by the Fundamental Research Funds for the Central Universities (NO. 3332018010).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- He H, Long Y, Zhou X, et al. Oxygen-Flow-Pressure Targets for Resuscitation in Critical Hemodynamic Therapy. Shock 2018;49:15-23. [Crossref] [PubMed]
- Groeneveld AB. Interpreting the venous-arterial PCO2 difference. Crit Care Med 1998;26:979-80. [Crossref] [PubMed]
- Dres M, Monnet X, Teboul JL. Hemodynamic management of cardiovascular failure by using PCO(2) venous-arterial difference. J Clin Monit Comput 2012;26:367-74. [Crossref] [PubMed]
- van Beest PA, Lont MC, Holman ND, et al. Central venous-arterial pCO(2) difference as a tool in resuscitation of septic patients. Intensive Care Med 2013;39:1034-9. [Crossref] [PubMed]
- Cuschieri J, Rivers EP, Donnino MW, et al. Central venous-arterial carbon dioxide difference as an indicator of cardiac index. Intensive Care Med 2005;31:818-22. [Crossref] [PubMed]
- Ospina-Tascon GA, Bautista-Rincon DF, Umana M, et al. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care 2013;17:R294. [Crossref] [PubMed]
- Vallee F, Vallet B, Mathe O, et al. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Med 2008;34:2218-25. [Crossref] [PubMed]
- Mallat J, Pepy F, Lemyze M, et al. Central venous-to-arterial carbon dioxide partial pressure difference in early resuscitation from septic shock: a prospective observational study. Eur J Anaesthesiol 2014;31:371-80. [Crossref] [PubMed]
- Bakker J, Vincent JL, Gris P, et al. Veno-arterial carbon dioxide gradient in human septic shock. Chest 1992;101:509-15. [Crossref] [PubMed]
- Du W, Liu DW, Wang XT, et al. Combining central venous-to-arterial partial pressure of carbon dioxide difference and central venous oxygen saturation to guide resuscitation in septic shock. J Crit Care 2013;28:1110.e1111-5. [Crossref] [PubMed]
- Troskot R, Šimurina T, Žižak M, et al. Prognostic value of venoarterial carbon dioxide gradient in patients with severe sepsis and septic shock. Croat Med J 2010;51:501-8. [Crossref] [PubMed]
- Muller G, Mercier E, Vignon P, et al. Prognostic significance of central venous-to-arterial carbon dioxide difference during the first 24 hours of septic shock in patients with and without impaired cardiac function. Br J Anaesth 2017;119:239-48. [Crossref] [PubMed]
- He H, Long Y, Liu D, et al. The Prognostic Value of Central Venous-to-Arterial CO2 Difference/Arterial-Central Venous O2 Difference Ratio in Septic Shock Patients with Central Venous O2 Saturation >/=80. Shock (Augusta, Ga) 2017;48:551-7. [Crossref] [PubMed]
- Diaztagle Fernández JJ, Rodríguez Murcia JC, Sprockel Díaz JJ. Venous-to-arterial carbon dioxide difference in the resuscitation of patients with severe sepsis and septic shock: A systematic review. Med Intensiva 2017;41:401-10. [PubMed]
- Saludes P, Proença L, Gruartmoner G, et al. Central venous-to-arterial carbon dioxide difference and the effect of venous hyperoxia: A limiting factor, or an additional marker of severity in shock? J Clin Monit Comput 2017;31:1203-11. [Crossref] [PubMed]
- He HW, Liu DW, Ince C. Understanding elevated Pv-aCO2 gap and Pv-aCO2/Ca-vO2 ratio in venous hyperoxia condition. J Clin Monit Comput 2017;31:1321-3. [Crossref] [PubMed]
- Orbegozo Cortés D, Puflea F, Donadello K, et al. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvasc Res 2015;98:23-8. [Crossref] [PubMed]
- Teboul JL, Scheeren T. Understanding the Haldane effect. Intensive Care Med 2017;43:91-3. [Crossref] [PubMed]
- Saludes P, Proença L, Gruartmoner G, et al. In response to: "understanding elevated Pv-aCO2 gap and Pv-aCO2/Ca-vO2 ratio in venous hyperoxia condition J Clin Monit Comput 2017;31:1325-7. [Crossref] [PubMed]
- Mallat J, Mohammad U, Lemyze M, et al. Acute hyperventilation increases the central venous-to-arterial PCO2 difference in stable septic shock patients. Ann Intensive Care 2017;7:31. [Crossref] [PubMed]
- Vallet B, Teboul JL, Cain S, et al. Venoarterial CO(2) difference during regional ischemic or hypoxic hypoxia. J Appl Physiol (1985) 2000;89:1317-21. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Textoris J, Fouche L, Wiramus S, et al. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care 2011;15:R176. [Crossref] [PubMed]
- Vallet B, Pinsky MR, Cecconi M. Resuscitation of patients with septic shock: please “mind the gap”! Intensive Care Med 2013;39:1653-5. [Crossref] [PubMed]
- Ospina-Tascon GA, Umana M, Bermudez WF, et al. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med 2016;42:211-21. [Crossref] [PubMed]
- Edul VS, Ince C, Vazquez AR, et al. Similar microcirculatory alterations in patients with normodynamic and hyperdynamic septic shock. Ann Am Thorac Soc 2016;13:240-7. [PubMed]
- Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care 2015;19 Suppl 3:S8. [PubMed]
- Dubin A, Pozo MO. Shedding light on venoarterial PCO2 gradient. Ann Intensive Care 2017;7:41. [Crossref] [PubMed]


