
Effects of pulsatile minimal invasive extracorporeal circulation on fibrinolysis and organ protection in adult cardiac surgery—a prospective randomized trial
Introduction
Cardiac surgeries are commonly performed after placing the patient on cardiopulmonary bypass (CPB). Besides classic heart-lung machines, minimal invasive extracorporeal circulation (MiECC) has proven to be an alternative which tries to minimize detrimental effects of extracorporeal circulation (ECC) on inflammation, hemostasis, and hemolysis (1-3). Some MiECC systems support pulsatile flow in addition to the default laminar flow. Pulsation is supposed to improve perfusion of critical organs, most notably brain and kidneys (4,5).
Acute kidney injury (AKI) is a common adverse effect after cardiac surgery, causing a rapid decrease of renal function within hours or days (6). As AKI is associated with a high mortality, an assessment of risk factors and predictive scoring may help to identify high-risk patients. Early detection of renal damage is still critical to initiate measures to prevent AKI in a timely manner. In contrast to evaluating glomerular filtration rate, measurement of serum or urinary markers may help to detect renal damage even before function is impaired. Serum or urinary Neutrophil gelatinase associated lipocalin (NGAL) indicates tubular damage within 4-6 hours post op (7,8). Along the same lines, urinary alpha glutathione S-transferase (alpha-GST) (9), urinary kidney injury molecule 1 (KIM-1) (10), and urinary liver fatty acid binding protein (L-FABP) have been reported to be rapid and useful markers of renal damage (11).
Detecting post-operative neuronal damage by cognitive tests is time-consuming and difficult to standardize. As in renal damage, biomarkers were tested to predict neuronal damage before functional impairments can be assessed. Neuron-specific enolase (NSE) and S100 have been suggested as markers of cerebral damage after cardiac surgery (12,13). Kynurenine, which is generated from the amino acid tryptophan by indoleamine 2,3-dioxygenase, has several breakdown products whose serum levels have been reported to correlate with post-operative cognitive impairment (14).
ECC is known to affect several aspects of hemostasis. One of them is the fibrinolytic balance. Tissue plasminogen activator (tPA) is a serine protease which generates plasmin from plasminogen. tPA is expressed in vascular endothelium and in neuronal cells. The active enzyme degrades fibrin and thus catalyzes the breakdown of blood clots as a prerequisite of tissue regeneration. Plasminogen activator inhibitor-1 (PAI-1) is a naturally occurring inhibitor of tPA (15). In healthy subjects, both proteins are at an equilibrium. Excess PAI-1 may induce myocardial infarction via coronary artery thrombosis (16), whereas excess tPA may degrade non-specific targets such as neuronal proteins (17). Therefore, maintenance of a PAI-1:tPA equilibrium is critical for reducing post-operative neurological problems.
Aĝirbaşli et al. have demonstrated that pulsatile operation of MiECC prevents an unwanted excess of tPA activity after pediatric cardiac surgery (18). The present prospective randomized study investigated pulsatile vs. non-pulsatile MiECC in adult cardiac surgery patients. The primary end point was a difference in the PAI-1:tPA ratio during CPB between groups. Secondary end points included differences in markers of renal and neuronal damage.
Methods
Study design
The present study was designed as a prospective randomized trial which compared the treatment group (pulsatile MiECC, p group) against a control group (non-pulsatile MiECC, np group). Treatments were assigned in a block-random design to achieve similar group sizes. Random sequences were generated in the Institute of Clinical Epidemiology and Biostatistics, University of Ulm, Germany, using the software R (19). Group assignments were provided in sealed envelopes and were opened by the perfusionist after the patient had provided written informed consent.
Patients
Patients of the Department of Cardiothoracic and Vascular Surgery, University of Ulm Medical Center, Germany, scheduled for isolated elective coronary bypass grafting with at least three required and realizable downstream anastomoses and MiECC were eligible. Exclusion criteria comprised age below 18 years, inability to provide written informed consent, preoperative catecholamines, terminal renal insufficiency, and prior cerebral ischemia.
Surgical techniques and MiECC
Anesthesia was initiated by intravenous administration of fentanyl, etomidat, midazolam, and pancuronium. Anesthesia was maintained by inhaled sevoflurane before and after CPB, and by intravenous sufentanil and disoprivan during CPB. Patients were anticoagulated with heparin using a target activated clotting time of 400 s just before MiECC was initiated. MiECC (MINI.SYSTEM 1.0 including a deltastream DP3 pump, Medos, Stolberg, Germany; Bioline coated tubing set, Maquet, Hirrlingen, Germany) was primed with 600 mL of Ringer solution. In the group np, laminar flow was used according to the flow and pressure requirements of the patient, usually 2.4 L per square meter of body surface and a mean arterial pressure of 65 mmHg. In the group p, pulsation at a frequency of 40 bpm was superimposed on the laminar flow. To this end, the rotational speed of the pump was transiently increased by 2,500 rpm over the baseline speed once per simulated cardiac cycle, resulting in a systolic duration of 35% of the cycle. After administering blood cardioplegia (20), distal anastomoses were created. Proximal anastomoses were created during partial clamping of the aorta. Anticoagulation was reversed with protamine. Patients were allowed to cool passively to no less than 35 °C and were rewarmed at the end of surgery.
Assessment of blood and urinary markers
Blood and urine samples were collected prior to skin incision (time point A), immediately after cross-clamping (B), 30 min after cross-clamping (C), five min after cross clamp removal (D), 20 min after cross clamp removal (E), five min after weaning off CPB (F), upon ICU arrival (G), 12 h after ICU arrival (H), and 72 h after ICU arrival (I). Blood was drawn from a central venous catheter (CVC) except during CPB (B through E) when blood was collected from the venous line. Blood was drawn from a peripheral vein at time point I if the CVC had already been removed. Urine was collected through a urinary catheter except at time point I where spontaneous urine was used in the absence of a urinary catheter. Serum and urine sample aliquots were stored at −80 °C. Some aliquots of each urine sample were stored in alpha-GST stabilizing buffer (Argutus Medical, Dublin, Ireland) at −80 °C.
Serum levels of C-reactive protein (CRP), S100, NSE, D-dimers, urea, serum and urinary levels of creatinin, and glomerular filtration rate (GFR) were determined by routine methods in the local Department of Clinical Chemistry. One mL of each urine sample was used for routine analysis. Owing to the low remaining volume in many urine samples, equal volumes of urine samples taken at times B through F were pooled (Bp). PAI-1 (eBioscience, Frankfurt/Main, Germany), tPA (eBioscience), NGAL (Bioporto, Hellerup, Denmark), KIM-1 (BioAssay Works, Ijamsville, MD, USA), alpha-GST (EKF Diagnostics, Dublin, Ireland), and L-FABP (CMIC, Tokyo, Japan) were determined by commercial enzyme-linked immuno sorbent assay (ELISA) kits according to the manufacturers’ instructions. Kynurenine was measured by a chromogenic method (21). Hemolysis was assessed by measuring serum levels of free hemoglobin with an Allen correction (22).
Statistical analysis
Sample size calculation (NQUERY; Statistical Solutions, Cork, Ireland) was based on the effect size and dispersion of the PAI-1:tPA ratios reported by (18) and suggested three patients per group. This was increased to twenty patients per group as the effect sizes of renal and neuronal markers were expected to be lower. PAI-1/tPA were calculated per patient, and the data were aggregated afterwards. Normality of data was analyzed with the Shapiro-Wilk test and QQ plots. Normally distributed data are reported as mean (standard deviation); non-normally data are presented as median (interquartile range). Perioperative numeric data were compared with two-sample t-tests and two-sample Wilcoxon-tests for normally and non-normally distributed data, respectively. Binary data were compared with Fisher’s exact test. Ordinal data were compared with the Cochran-Armitage test. Time courses of serum and urinary marker levels were assessed with mixed model analysis, using pulsation and collection time as factors, and patients as source of random effects. Time-dependent changes against baseline within groups were assessed by Dunnett’s post-test. Pulsation-dependent changes at individual time points were evaluated with t-tests and Wilcoxon-tests as described above. P values below 0.05 were assumed to indicate statistically significant differences. Statistical analyses were calculated with R and its nlme and multcomp packages for mixed model analysis and multiple comparison post-tests, respectively. Power calculations were done with the pwr package, using a significance level of 0.05.
Results
Patient characteristics
Of the 40 patients originally enrolled in the study, three had to be excluded. One patient assigned to the np group was operated on with pulsation. Two patients were erroneously included although they did not meet all inclusion criteria. Additional randomization was performed to raise the number of included patients to 40 (np: n=19; p: n=21). The patient characteristics (Table 1) indicate no confounding differences between groups with the exception of preoperative medication; Clopidogrel was taken only by patients of the pulsatile group (n=8), and preoperative diuretics were more common in this group (np: n=1, p: n=8).

Full table
Intra- and post-operative data
Pulsation in the p group was characterized by an average pulse amplitude of 25 mmHg (SD: 3). Minimum and maximum amplitudes amounted to 12 mmHg (SD: 3) and 38 mmHg (SD: 4), respectively. There were no significant differences between groups in terms of MAP, minimum diastolic, and maximum systolic blood pressures (Table 2). Bypass times, cross clamp times, and numbers of distal anastomoses were also comparable. Pulsatile perfusion led to an increased intraoperative urinary excretion, an increased cumulative volume uptake 24 h post-operative, and decreased blood losses within 12 and 24 h post-operative.
Full table
There were no cases of stroke or kidney failure until discharge in either group. None of the patients died within the first 12 months after surgery. Post-operative courses were comparable. The slightly later discharge in the np group [10 (9 to 12) vs. 9 (9 to 11), P=0.276] was not significant.
Fibrinolytic markers
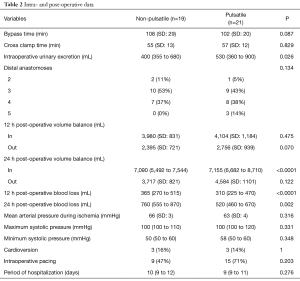
Time courses of markers of fibrinolysis are shown in Figure 1. PAI-1 levels and the PAI-1/tPA ratio were below baseline during ECC but approached baseline levels again after weaning from CPB. tPA increased above baseline during CPB but returned to baseline at 72 h post-operative. Pulsation did not significantly affect overall time courses of PAI-1 (P=0.150), PAI-1 (P=0.290), and PAI-1/tPA (P=0.640). Pairwise comparisons of non-pulse and pulse data at individual time point indicate differences of tPA levels only at time points D (P=0.030) and E (P=0.0485); these are not considered significant after correction for multiple testing, just like all other comparisons of PAI-1 and PAI-1/tPA levels at individual time points.
Apparent effects on fibrinolytic balance were present only at time points C and D. The calculated powers of the PAI-1/tPA ratio were 0.15 and 0.05, respectively.
Renal markers
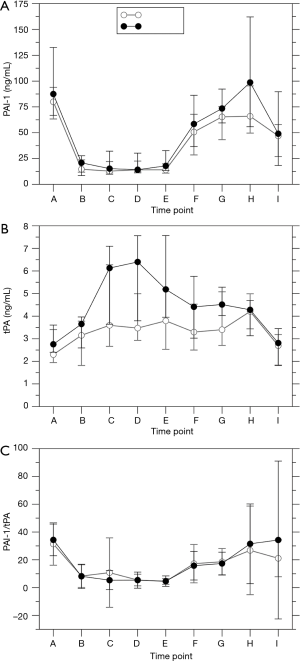
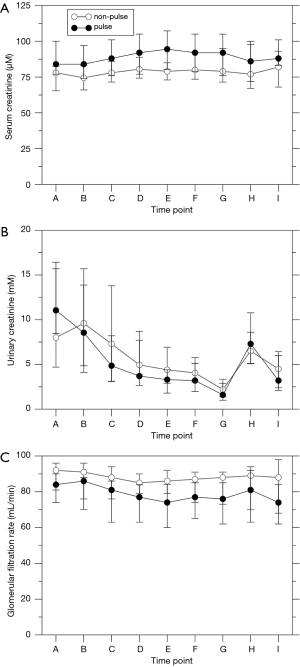
Urinary levels of markers of renal damage followed established time courses (Figure 2), reaching a maximum either 12 or 72 h post-operative. alpha-GST levels in the np (P=0.013) and in the p group (P=0.001), KIM-1 levels in both groups (P<0.0001), L-FABP levels in the np (P=0.006) and in the p group (P<0.0001), and NGAL levels in the p group (P=0.007) changed over time, whereas sample time did not influence NGAL levels in the np group (P=0.935). Pulsation did not significantly influence the levels of alpha-GST (P=0.436), KIM-1 (P=0.964), L-FABP (P=0.303), and NGAL (P=0.547). Sample time influenced serum creatinine levels in the p group (Figure 3; P=0.006), with an intraoperative maximum at E, but not in the np group (P=0.10). Urinary creatinine levels decreased over time in both groups (P<0.0001). GFR decreased slightly over time in both groups (np: P=0.033; p: P<0.0001) but was not affected by pulsation (P=0.150). Acute kidney insufficiency occurred in 1/19 patients in the np group and in 4/21 patients in the p group (P=0.345).
Neuronal markers
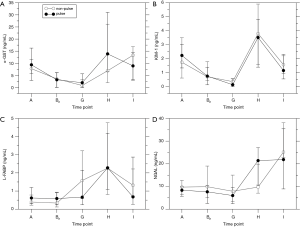
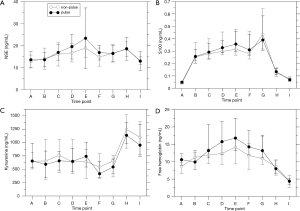
The markers of neuronal damage NSE and S100 were elevated intraoperatively in both groups (P<0.0001; Figure 4) but there was no influence of pulsation (NSE: P=0.281; S100: P=0.626). The post-operative increase in kynurenine levels was significant only in the np group (P<0.0001; p: P=0.354). Pulsation did not have a significant effect (P=0.415).
Hemolysis
Free hemoglobin levels as a marker of hemolysis were elevated intraoperatively in both groups (Figure 4D; P<0.0001). Although there was no overall influence of pulsation (P=0.209), levels were higher in the p group preoperatively (P=0.012) and after weaning off ECC (P=0.036) which is not considered significant after correction for multiple testing.
Discussion
The present study compared pulsatile and non-pulsatile operation of MECC in low-risk CABG patients. Although pulsation improved renal perfusion and reduced post-operative blood losses, there were no substantial effects on markers of renal and neuronal damage and on the fibrinolytic balance.
Patients of both study groups were comparable in their preoperative health status. However, more patients of the p group used clopidogrel and diuretics preoperatively. Potential effects of these drugs on fibrinolysis (23) and on kidney function, respectively, must be taken into consideration. There were no high-risk patients included as per the inclusion and exclusion criteria.
Pulsation had only few effects on the progress and outcomes of operations. CPB times and cross clamp times were comparable. Pulsation increased maximum blood pressure during systole and decreased minimum blood pressure during diastole. There was no effect on mean arterial pressure which makes both groups comparable. Intraoperative urinary excretion was elevated in the p group, indicating an increased renal blood flow by pulsation, and was compensated by an increased volume intake within the first 24 h post-operative. Both effects have previously been reported in other studies (24,25), but preoperative diuretics mainly in the p group may have contributed to these results. Pulsatile MiECC operation was also associated with lower blood losses within 12 and 24 h post-operative whereas there was no such benefit of a pulsatile conventional heart-lung machine (26). Therefore, pulsation may further enhance the reduction of blood losses observed in MiECC vs. conventional ECC (27). At present there is no simple model to explain the reduced blood losses in the p group in spite of the higher number of patients taking clopidogrel preoperatively. Several potential consequences of pulsatile perfusion, such as reduced subclinical activation of coagulation during CPB resulting in a better preservation of coagulation factors, better preservation of the shear-stress-regulated endothelial function, reduced post-operative vascular resistance, and better post-operative elimination of excess volume, may have contributed to attenuate post-operative blood losses. Post-operative courses of the patients were not affected by pulsation.
Pulsation did not affect fibrinolytic balance. The intraoperative drop of PAI-1 levels to less than one fifth of the baseline values cannot be explained by dilution effects alone. The response is very rapid and can best be explained by a consumption of the inhibitor which is replenished after weaning off CPB. There is a noticeable 2.7-fold increase of tPA in the p group while the aorta is clamped until 20 min after opening the cross clamp. The increase in the np group is less than 1.4-fold. The corrected p values do not indicate a significant effect, but the power is too low to safely exclude a significant effect. The disparity in preoperative clopidogrel consumption (0/19 in the np group, 8/21 in the p group) must be considered as a confounding factor. On the other hand, the PAI-1/tPA ratios of both groups run almost parallel and do not suggest an influence of pulsation. The strongly asymmetric distribution of intraoperative p group PAI-1 data partly explains the apparent congruence of PAI-1/tPA data in spite of the potential influence of pulsation on tPA levels. These results are in contrast to a previously published study in pediatric patients (18). In that study, the PAI-1/tPA ratio of the pulsatile group was higher at 24 h post-operative which was caused by an increased PAI-1 release with no difference in tPA release. In addition to potential effects of preoperative clopidogrel, which is unlikely to be administered to pediatric patients, differences in vascular compliance may explain the different results in patients of young and advanced age. Both tPA (28) and PAI-1 (29) secretion was shown to be affected by mechanical stimuli ex vivo. In addition, fibrinolytic capacity appears to increase with age in healthy subjects (30). On the other hand, common risk factors of cardiovascular diseases, such as hypertension (31), obesity (32), and diabetes (33), have been shown to impair fibrinolytic capacity. Common medications to treat these risk factors were shown to affect PAI-1 release as well (33).
Urinary alpha-GST and KIM-1 levels showed a noticeable decrease intraoperatively which was reversed only within the next 12 h. Urinary L-FABP and NGAL levels increased only post-operatively. Time courses resembled that of a previous trial of our department (34). There was no influence of pulsation on these markers, irrespective of the increased urinary excretion during CPB in the p group. This supports the hypothesis that the increased intraoperative urinary secretion in the p group can be explained by an improved blood perfusion rather than by an improved protection against renal damage. However, these data contradict a previous study that reported decreased post-operative NGAL and IL-18 levels after pulsatile ECC (35). Serum and urinary creatinine levels, GFR, and the post-operative ocurrence of acute kidney injury were not significantly affected by pulsation. These data confirm a recent meta-analysis that found no effect of pulsation on post-operative NGAL levels and on acute kidney injury (36).
The levels of markers of neuronal damage, NSE, S100, and kynurenine, showed time-dependent changes but were not affected by pulsation. NSE time courses were comparable to previously published data (37), peaking near the end of CPB and returning to baseline at the end of the observation period. However, the study of Gao et al. found similar time courses of S100 levels whereas the peak in the present study was upon ICU arrival. This difference may be related to the use of hypothermia in the previous study. Hemolysis also peaked near the end of CPB. There was a tendency towards increased hemolysis by pulsation although the difference was not significant. Previous studies did not find effects of pulsatile ECC on post-operative neurological outcomes (38,39) and on NSE and S100 levels (39).
The interpretation of the present data is hampered by the limited number of patients. Although the number of study patients was six-fold higher than suggested by the power calculation based on pediatric data, the assumption of a comparable effect size of the PAI-1/tPA ratios in adult patients turned out to be wrong. Using the number of patients included, the power of PAI-1/tPA did not exceed 0.15 at any given time point. The study was not powered for the remaining markers. Therefore, an influence of pulsation cannot be safely excluded, although the marker levels of the present study do not point towards any differences except in intraoperative tPA levels. Comparisons with other studies need some caution as the parameters of pulsatile flow have not been standardized. In the present study, the pump settings were chosen to maximize pulse amplitude at the given flow and mean arterial pressure. This, however, is not necessarily identical with the settings required to maximize pulsatile energy transfer in terms of energy equivalent pressure (40). The authors have since started to analyze their MiECC system to optimize the latter (41). Finally, effects of pulsation may be more pronounced in surgeries which are longer than the low-risk CABG procedures of the present trial.
In conclusion, the present study did not find evidence for a beneficial effect of pulsation on markers of fibrinolysis, renal damage, and neuronal damage. However, pulsatile perfusion increased intraoperative urinary secretion and reduced post-operative blood losses.
Acknowledgments
The authors wish to thank the surgical staff, the operating theater staff, and the technical assistants for their help in acquiring and analyzing the blood and urine samples.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The trial was approved by the ethics committee of the University of Ulm, Germany (file no. 84/14). Patients willing to participate in the trial were required to provide written informed consent before enrollment.
References
- Philipp A, Foltan M, Thrum A, et al. MECC – ein minimiertes EKZ-System für ACB-Operationen. Kardiotechnik 2001;1:14-9.
- Wiesenack C, Liebold A, Philipp A, et al. Four years' experience with a miniaturized extracorporeal circulation system and its influence on clinical outcome. Artif Organs 2004;28:1082-8. [Crossref] [PubMed]
- Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 2016;22:647-62. [Crossref] [PubMed]
- Undar A, Masai T, Beyer EA, et al. Pediatric physiologic pulsatile pump enhances cerebral and renal blood flow during and after cardiopulmonary bypass. Artif Organs 2002;26:919-23. [Crossref] [PubMed]
- Kocakulak M, Aşkin G, Kuçukaksu S, et al. Pulsatile flow improves renal function in high-risk cardiac operations. Blood Purif 2005;23:263-7. [Crossref] [PubMed]
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 2006;1:19-32. [Crossref] [PubMed]
- Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005;365:1231-8. [Crossref] [PubMed]
- de Geus HRH, Ronco C, Haase M, Jacob L, Lewington A, et al. The cardiac surgery-associated neutrophil gelatinase-associated lipocalin (CSA-NGAL) score: A potential tool to monitor acute tubular damage. J Thorac Cardiovasc Surg 2016;151:1476-81. [Crossref] [PubMed]
- McMahon BA, Koyner JL, Murray PT. Urinary glutathione S-transferases in the pathogenesis and diagnostic evaluation of acute kidney injury following cardiac surgery: a critical review. Curr Opin Crit Care 2010;16:550-5. [Crossref] [PubMed]
- Wang JJ, Chi N, Huang T, et al. Urinary biomarkers predict advanced acute kidney injury after cardiovascular surgery. Crit Care 2018;22:108. [Crossref] [PubMed]
- Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int 2008;73:465-72. [Crossref] [PubMed]
- Georgiadis D, Berger A, Kowatschev E, et al. Predictive value of S-100beta and neuron-specific enolase serum levels for adverse neurologic outcome after cardiac surgery. J Thorac Cardiovasc Surg 2000;119:138-47. [Crossref] [PubMed]
- Rasmussen LS, Christiansen M, Eliasen K, et al. Biochemical markers for brain damage after cardiac surgery -- time profile and correlation with cognitive dysfunction. Acta Anaesthesiol Scand 2002;46:547-51. [Crossref] [PubMed]
- Forrest CM, Mackay GM, Oxford L, et al. Kynurenine metabolism predicts cognitive function in patients following cardiac bypass and thoracic surgery. J Neurochem 2011;119:136-52. [Crossref] [PubMed]
- Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost 2005;3:35-45. [Crossref] [PubMed]
- Eren M, Painter CA, Atkinson JB, et al. Age-dependent spontaneous coronary arterial thrombosis in transgenic mice that express a stable form of human plasminogen activator inhibitor-1. Circulation 2002;106:491-6. [Crossref] [PubMed]
- Knottnerus ILH, Govers-Riemslag JWP, Hamulyak K, et al. Endothelial activation in lacunar stroke subtypes. Stroke 2010;41:1617-22. [Crossref] [PubMed]
- Aĝirbaşli MA, Song J, Lei F, et al. Comparative Effects of Pulsatile and Nonpulsatile Flow on Plasma Fibrinolytic Balance in Pediatric Patients Undergoing Cardiopulmonary Bypass. Artif Organs 2014;38:28-33. [Crossref] [PubMed]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2014.
- Calafiore AM, Teodori G, Bosco G, et al. Intermittent antegrade warm blood cardioplegia in aortic valve replacement. J Card Surg 1996;11:348-54. [Crossref] [PubMed]
- Kaden J, Abendroth D, Völp A, et al. Causes and prognostic value of pre-transplant elevated kynurenine level in kidney allograft recipients. Ann Transplant 2014;19:51-9. [Crossref] [PubMed]
- Noe DA, Weedn V, Bell WR. Direct spectrophotometry of serum hemoglobin: an Allen correction compared with a three-wavelength polychromatic analysis. Clin Chem 1984;30:627-30. [PubMed]
- Spinthakis N, Farag M, Gue YX, et al. Effect of P2Y inhibitors on thrombus stability and endogenous fibrinolysis. Thromb Res 2019;173:102-8. [Crossref] [PubMed]
- Haines NM, Wang S, Kunselman A, et al. Comparison of pumps and oxygenators with pulsatile and nonpulsatile modes in an infant cardiopulmonary bypass model. Artif Organs 2009;33:993-1001. [Crossref] [PubMed]
- Undar A, Henderson N, Thurston GB, et al. The effects of pulsatile versus nonpulsatile perfusion on blood viscoelasticity before and after deep hypothermic circulatory arrest in a neonatal piglet model. Artif Organs 1999;23:717-21. [Crossref] [PubMed]
- Driessen JJ, Fransen G, Rondelez L, et al. Comparison of the standard roller pump and a pulsatile centrifugal pump for extracorporeal circulation during routine coronary artery bypass grafting. Perfusion 1991;6:303-11. [Crossref] [PubMed]
- Gerritsen WB, van Boven WJ, Wesselink RM, et al. Significant reduction in blood loss in patients undergoing minimal extracorporeal circulation. Transfus Med 2006;16:329-34. [Crossref] [PubMed]
- Sjögren LS, Doroudi R, Gan L, et al. Elevated intraluminal pressure inhibits vascular tissue plasminogen activator secretion and downregulates its gene expression. Hypertension 2000;35:1002-8. [Crossref] [PubMed]
- Cheng JJ, Chao YJ, Wung BS, et al. Cyclic strain-induced plasminogen activator inhibitor-1 (PAI-1) release from endothelial cells involves reactive oxygen species. Biochem Biophys Res Commun 1996;225:100-5. [Crossref] [PubMed]
- Gudnason T, Hrafnkelsdóttir T, Wall U, et al. Fibrinolytic capacity increases with age in healthy humans, while endothelium-dependent vasodilation is unaffected. Thromb Haemost 2003;89:374-81. [Crossref] [PubMed]
- Hrafnkelsdóttir T, Wall U, Jern C, et al. Impaired capacity for endogenous fibrinolysis in essential hypertension. Lancet 1998;352:1597-8. [Crossref] [PubMed]
- Van Guilder GP, Hoetzer GL, Smith DT, et al. Endothelial t-PA release is impaired in overweight and obese adults but can be improved with regular aerobic exercise. Am J Physiol Endocrinol Metab 2005;289:E807-13. [Crossref] [PubMed]
- Grant MB, Fitzgerald C, Guay C, et al. Fibrinolytic capacity following stimulation with desmopressin acetate in patients with diabetes mellitus. Metabolism 1989;38:901-7. [Crossref] [PubMed]
- Deininger S, Hoenicka M, Müller-Eising K, et al. Renal Function and Urinary Biomarkers in Cardiac Bypass Surgery: A Prospective Randomized Trial Comparing Three Surgical Techniques. Thorac Cardiovasc Surg 2016;64:561-8. [PubMed]
- Adademir T, Ak K, Aljodi M, et al. The effects of pulsatile cardiopulmonary bypass on acute kidney injury. Int J Artif Organs 2012;35:511-9. [Crossref] [PubMed]
- Nam MJ, Lim CH, Kim H, et al. A Meta-Analysis of Renal Function After Adult Cardiac Surgery With Pulsatile Perfusion. Artif Organs 2015;39:788-94. [Crossref] [PubMed]
- Gao F, Harris DN, Sapsed-Byrne S. Time course of neurone-specific enolase and S-100 protein release during and after coronary artery bypass grafting. Br J Anaesth 1999;82:266-7. [Crossref] [PubMed]
- Murkin JM, Martzke JS, Buchan AM, et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. I. Mortality and cardiovascular morbidity. J Thorac Cardiovasc Surg 1995;110:340-8. [Crossref] [PubMed]
- Öztürk S, Saçar M, Baltalarlı A, et al. Effect of the type of cardiopulmonary bypass pump flow on postoperative cognitive function in patients undergoing isolated coronary artery surgery. Anatol J Cardiol 2016;16:875-80. [PubMed]
- Shepard RB, Simpson DC, Sharp JF. Energy equivalent pressure. Arch Surg 1966;93:730-40. [Crossref] [PubMed]
- Dürr A, Kunert A, Albrecht G, et al. Hemodynamic energy during pulsatile extracorporeal circulation using flexible and rigid arterial tubing: a reassessment. Perfusion 2019;34:297-302. [Crossref] [PubMed]


