
Venous-to-arterial pCO2 difference in high-risk surgical patients
Introduction
Individualized hemodynamic optimization during high-risk surgery is an essential key to patient care. Several studies have demonstrated that such strategies improve the postoperative course by reducing morbidity and mortality. Hemodynamic optimization is a preventive strategy aiming to adapt oxygen delivery (DO2) to oxygen consumption (VO2) to avoid tissue hypoperfusion during surgery (1). This strategy is based on optimization of blood pressure (fluid and/or vasopressor), cardiac output (CO) (fluid and/or inotrope), and perfusion parameters such as central venous oxygen saturation (ScVO2) or arterial lactate. Nevertheless, normalizing systemic hemodynamic parameters and ScVO2 does not guarantee adequate tissue perfusion, and a substantial number of patients still progress to multiple organ failure and death. Although blood lactate concentration was initially described as a surrogate marker of tissue hypoperfusion, an elevated lactate value may be associated with adrenergic stimulation and surgical stress (2).
Recently, the difference between venous carbon dioxide and arterial carbon dioxide pressure (pCO2 gap) has been described as a parameter reflecting tissue hypoperfusion in critically ill patients who are insufficiently resuscitated (3). Similarly, the pCO2 gap/CavO2 ratio has been described as an indicator of the respiratory quotient, thus the relationship between DO2 and VO2 (4). Most of the knowledge about the pCO2 gap and the pCO2 gap/CavO2 ratio comes from studies in the literature on animal models or intensive care unit (ICU) patients (5). These parameters have been demonstrated to be associated with several variables of tissue perfusion, and most importantly with outcomes (mortality, morbidity). To date, publications pertaining to the operative setting have been sparse.
The purpose of the present review is to discuss clinical evidence of the usefulness of the pCO2 gap and CO2-O2 derived parameters in the operating room. We will then discuss the results according to the type of surgery (cardiac vs. non-cardiac) and propose clinical use.
Physiological background
According to the Fick equation, CO2 elimination (VCO2) equals the product of the difference between venous blood CO2 content (CvCO2), arterial blood CO2 content (CaCO2), and CO [VCO2 = CO × (CvCO2 − CaCO2)]. Because there is a linear association between CO2 content and CO2 pressure, the pCO2 gap may be expressed as: pCO2 gap = K * VCO2/CO. Therefore, the pCO2 gap could be associated with CO2 generation and CO. As CO2 is much more soluble than O2, it represents a very sensitive marker of tissue hypoxia (6). Since the pCO2 gap depends on CO and VCO2, it represents an indicator of the capacity of venous blood to eliminate CO2 generated by peripheral tissues, and thus the adequacy of blood flow during shock states. Interestingly, an inverse curvilinear relationship between the pCO2 gap and CO has been described, highlighting the importance of blood flow on venous CO2 accumulation (7,8).
Several studies on septic shock have found that an increase in CO with fluid expansion is associated with a decrease in the pCO2 gap compared to increased CO (9). For a constant production of CO2, the increase in CO is coupled with an increased arterial blood volume having a low CO2 content passing through the tissue, producing a washout effect and lowering the venous CO2 content. Another factor in the lowering of the pCO2 gap is the effect of blood pH on the relationship between pCO2 and total blood CO2 content. This relationship is shifted to the right, with a pH decrease resulting in an increased pCO2 gap for the same value of CvCO2. Consequently, an increase in CO will be associated with lower pCO2 gap if the tissue acid production is decreased by the improvement in oxygen supply (10). Finally, the mechanisms implicated in the elevation of the pCO2 gap during shock states are not completely understood, and interpretation of the pCO2 gap could sometimes be difficult.
The ratio between the pCO2 gap and the arterial-venous oxygen difference (pCO2 gap/CavO2 ratio) has also been described (11,12). Under situations of tissue hypoxia, we can observe that a decreased VO2 is associated with decreased aerobic CO2 generation, whereas anaerobic CO2 generation can still arise. Knowing that the VCO2 is being reduced less than the VO2, we can observe a rise in the VCO2/VO2 ratio (i.e., the respiratory quotient). Studies have demonstrated that the pCO2 gap/CavO2 ratio can be used as an indicator of the presence of overall tissue hypoxia in critically ill patients (13). Mekontso-Dessap and colleagues demonstrated, in a retrospective ICU cohort, that the pCO2 gap/CavO2 ratio may be a substitute for the respiratory quotient and blood lactate. The pCO2 gap/CavO2 ratio was able to predict the presence of hyperlactatemia (4). Subsequently, Monnet and colleagues demonstrated that this ratio was able to predict an increase in VO2 following fluid expansion in ICU patients. The pCO2 gap/CavO2 ratio was able to better predict the presence of VO2/DO2 dependency phenomenon than blood lactate and ScVO2 (14). In 2013, Vallet and colleagues proposed an interpretation of different shock states based on the analysis of blood lactate and O2-CO2 derived parameters (15) (Table 1).
Clinical relevance of the pCO2 gap in high-risk non-cardiac surgical patients
Several observational studies have been conducted in patients undergoing non-cardiac surgery. A prospective study on 51 elective neurosurgical patients evaluated the correlation between the pCO2 gap and CO. The authors demonstrated a close inverse correlation between CO and the pCO2 gap for both central and mixed venous gas samples. They concluded that the pCO2 gap could represent a useful parameter for CO assessment, and could be utilized in a neurosurgical practice involving postural changers (16). These authors did not evaluate outcomes. Futier and colleagues (17) conduced a retrospective study on 70 patients undergoing major abdominal surgery with an individualized goal-directed fluid replacement therapy. The pCO2 gap was measured every hour until the end of the surgery. Of the 70 patients, 34% developed postoperative septic complications. The authors demonstrate that high ScvO2 was not associated with postoperative complications, and that the pCO2 gap was the only parameter associated with complications (17). During the course of the surgery, the pCO2 gap was larger in patients with complications (7.8±2 vs. 5.6±2 mmHg, P<0.05) than in patients without complications. In addition, a pCO2 gap value >5 mmHg was able to predict postoperative complications with an area under the ROC curve (AUC) of 0.785 (95% CI: 0.74 to 0.83, P<0.05) (17). In patients with normal ScVO2, the pCO2 gap may be a useful complementary tool to identify patients who remain insufficiently optimized hemodynamically. Robin and colleagues later performed a prospective observational study in 115 high-risk non-cardiac surgery patients (mostly abdominal surgery) (18). The pCO2 gap was evaluated at admission to ICU, immediately after surgery. Seventy-eight patients (68%) developed postoperative complications. The pCO2 gap was significantly higher at ICU admission in patients who suffered from complications (8.7±2.8 vs. 5.1±2.6 mmHg, P<0.001). The pCO2 gap predicted the occurrence of postoperative complications, with an AUC of 0.86 (95% CI: 0.77 to 0.95) and a cut off value of 5.8 mmHg. Moreover, the pCO2 gap has a higher ability to predict postoperative complications than arterial lactate. Taking together the results of their studies, the authors concluded that “the PCO2 gap might be a useful and complementary tool to detect persistent tissue hypoperfusion that could be included as an additional step in the algorithms of early goal-directed therapy protocols” (18).
Apart from a retrospective study on 66 patients undergoing abdominal surgery, Silva and colleagues demonstrated an association between the pCO2 gap and mortality (19). A pCO2 gap of over 5 mmHg was predictive of mortality, with an AUC of 0.73 (95% CI: 0.61 to 0.84, P<0.05) (19). Recently, in a multicenter prospective observational study in non-cardiac surgery, our group demonstrated that the pCO2 gap and the pCO2 gap/CavO2 ratio were associated with the postoperative course (20). In summary, there is evidence supporting the association between the pCO2 gap, the pCO2 gap/CavO2 ratio, and postoperative morbidity and mortality. To date, no study has assessed the ability of hemodynamic protocols based on the pCO2 gap measurement to decrease postoperative complications.
Clinical relevance of the pCO2 gap in high-risk cardiac surgical patients
A study performed in the 90’s by Cavaliere and colleagues evaluated the pCO2 gap in 30 patients in the early postoperative hours following cardiac surgery (21). Of the 30 patients, 21 (70%) developed postoperative complications. The pCO2 gap was significantly higher at ICU admission in patients who suffered from complications (9±2 vs. 5±1 mmHg, P<0.001). By using a multiple linear regression analysis, the authors demonstrated that the pCO2 gap was associated to the body temperature, the paCO2 and the arterial mixed venous O2 content difference. The pCO2 gap was not associated to CO nor blood lactate (21). Based on the assumption that ScvO2 remains challenging as a tool to identify patients with adequate circulatory status, Habicher and colleagues performed a study in cardiac surgical patients with normal ScVO2 (22). The authors hypothesized that the pCO2 gap could serve as an additional parameter to evaluate the adequacy of tissue perfusion. A retrospective data analysis on 60 patients was performed. The patients had a ScvO2 ≥70% and were divided into 2 groups: the high-pCO2 gap group (≥8 mmHg) and the low-pCO2 gap group (<8 mmHg) (22). Patients with a high pCO2 gap had worse postoperative courses, with higher lactate levels and worse splanchnic functions. These findings were associated with need for longer mechanical ventilation and longer ICU stays. In 2016, a retrospective study that included 220 consecutive patients after elective cardiac surgery evaluated the association between the pCO2 gap and postoperative complications (23). The pCO2 gap was considered normal for a value less than 6 mmHg. The SOFA score and the mortality rate were higher in the low pCO2 gap group than in the high pCO2 gap group. Moreover, the pCO2 gap had a low ability to predict outcomes (23). Guinot and colleagues subsequently evaluated the association between the pCO2 gap during the ICU course following cardiac surgery and postoperative morbidity and mortality (24). Three hundred thirty-nine patients were enrolled in this prospective observational study. The pCO2 gap was not predictive of the development of major complications. Moreover, the pCO2 gap was poorly correlated with tissue perfusion parameters, and arterial lactate clearance (24).
Du and colleagues conducted an observational retrospective study to establish whether the pCO2 gap/CavO2 ratio could predict the hemodynamic response to resuscitation (25). Seventy-two patients undergoing cardiac surgery were analyzed. VO2 responders were defined by an increased VO2 of over 10%. The ratio appeared to be a reliable marker of overall anaerobic metabolism that predicted VO2 response. Abou-Arab and colleagues later analyzed the ability of the pCO2 gap/CavO2 ratio to predict an increase in VO2 upon fluid challenge in cardiac surgical patients (26). One hundred ten patients, consecutively admitted to a cardiothoracic ICU and in whom fluid expansion was performed, were included. VO2 responders were defined as patients showing more than 15% increase in VO2. Arterial pressure, CO, and arterial and venous blood gas levels were measured before and immediately after the fluid challenge. CO2-O2 derived variables were not predictive of VO2 changes following fluid challenge in this specific population (26). Only ScVO2 was poorly predictive of VO2 changes. The pCO2 gap/CavO2 ratio was not associate to arterial lactate. Interestingly, the authors observed a decrease in the pCO2 gap only in non-VO2 responder patients, suggesting a different pattern of microcirculatory alteration following cardiac surgery than in sepsis.
In summary, association between the pCO2 gap, pCO2 gap/CavO2 ratio, postoperative course and anaerobic metabolism is unclear in cardiac surgical area. Small and retrospective studies demonstrated positive results whereas larger cohort demonstrated negative results.
Divergence between non-cardiac surgery, cardiac surgery, and septic critically ill patients
When analyzing the literature, some explanations can be found regarding discrepancies in the published results. The most important is probably the type of surgery (21,27). Cardiac surgery with cardiopulmonary bypass is a specific physiologic situation that may be associated with factors altering the relationship between CO2-O2 derived content and pressure, VCO2, DO2, VO2, and tissue perfusion. On the contrary, non-cardiac major surgery is often abdominal surgery which may be more similar to the macro- and micro-circulatory disturbance observed in ICU patients (19). Ruokonen and colleagues have already studied the ability of the pCO2 gap to assess tissue perfusion in cardiac surgery patients by using a control group of abdominal surgery patients (27). According to this author, a pCO2 gap rise is frequent after cardiac surgery and better reflects an alteration of systemic and regional perfusion compared to tissue hypoxia (26). In this way, some studies did not demonstrate any association between the pCO2 gap, pCO2 gap/CavO2 ratio, arterial lactate and VO2 (21,26).
The relationship between CO2-O2 derived content and pressure depends on several parameters that can be altered in the operating theatre, specifically in cardiac surgery. Of these parameters, body temperature, alveolar ventilation, and hemodilution may be of importance. Van der Linden and colleague have demonstrated an increase in the pCO2 gap during acute hemorrhaging in anesthetized dogs. Hemorrhage was associated with a progressive increase in venous pCO2, with a corresponding widening of the pCO2 gap which was correlated with a blood lactate change (28). Nevertheless, hemodilution was demonstrated to have more complex effects on CO2-O2 derived variables than hemorrhage (29,30). During mechanical ventilation, alveolar ventilation may be associated with pCO2 changes. Mallat and colleagues and Morel and colleagues demonstrated similar results when analyzing the pCO2 gap during rising alveolar ventilation (31,32). Both studies demonstrated that rising alveolar ventilation is associated with an increased pCO2 gap. These changes were related to changes in VO2, systemic vasoconstriction, and variations in the PCO2/CO2 content relationship (31,32). By altering the metabolism and the PCO2/CO2 content relationship, body temperature can alter the adequacy of the pCO2 gap. Utoh and colleagues demonstrated, in cardiac surgical patients, that the two main factors associated with high pCO2 gap values were the duration of cardiopulmonary bypass surgery and the minimum rectal temperature. Cardiac surgery was shown to be associated with changes in metabolic rate, CO, and VO2 (15,21). Such alterations can occur throughout first postoperative hours.
The extent of microcirculation alterations caused by sepsis, surgery, and cardiopulmonary bypass may differ (33,34). Sepsis is normally associated with impaired microcirculatory regulation, decreased functional capillary index, absent/intermittent capillary flow, increased heterogeneity in the perfusion index, arteriovenous shunting, and cellular hypoxia (35). On the contrary, cardiopulmonary bypass is associated with many reversible alterations in microcirculation, including a decrease in microvascular perfusion, increased heterogeneity in the perfusion index and red blood cell velocity, and arteriovenous shunting (33,36). These changes are associated with alterations in the arteriovenous oxygen difference, VO2, and CO2 and O2 diffusion (37). During major abdominal surgery, the microvascular perfusion is not altered, and it is not associated with postoperative complications (38). Nevertheless, an impaired microvascular flow can appear during the postoperative period when patients suffer from complications (38). These changes are similar to those observed in sepsis (39).
Use of the pCO2 gap in high-risk surgical patients
One has to keep in mind that a high pCO2 gap may not necessarily indicate an alteration in tissue perfusion or a low flow state. Moreover, studies have demonstrated that a normal pCO2 gap does not preclude the presence of tissue hypoxia, and thus has poor sensitivity to detect tissue hypoxia (8,40). In patients with low CO and a normal arterial lactate value, the pCO2 gap was demonstrated to be increased (7). Keeping in mind these limitations and the fact that, to date, no randomized study using the pCO2 gap has been published, the pCO2 gap may be interpreted according to the type of surgery (cardiac vs. non-cardiac), medical situation (e.g., sepsis, haemorrhage, cardiogenic), and macro- and micro-hemodynamic parameters (e.g., CO, arterial lactate, ScVO2). The pCO2 gap may be considered as a parameter reflecting the ability of blood flow to remove the total CO2 produced by tissue rather than a marker of tissue dysoxia. Based on these interpretations, several authors have proposed algorithms. Among them, an algorithm based on the lactate value may be useful in the choice of therapeutic treatment for acute circulatory failure (Figure 1).
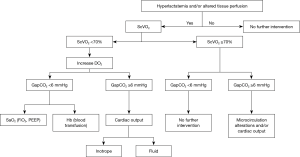
Conclusions
The pCO2 gap can be considered as a marker of CO adequacy for the overall metabolic demand that has been associated with the postoperative course in non-cardiac major surgery. The pCO2 gap may not always be a marker of tissue hypoxia. During hemodynamic treatment, the interpretation of the pCO2 gap may help physicians to understand which variables can be optimized. In cardiac surgery, results are inconsistent because of many factors altering the pCO2 gap interpretation. In surgical patients without any sign/parameter of tissue hypoperfusion, manipulating the pCO2 gap may be done with caution.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ariza M, Gothard JW, Macnaughton P, et al. Blood lactate and mixed venous-arterial PCO2 gradient as indices of poor peripheral perfusion following cardiopulmonary bypass surgery. Intensive Care Med 1991;17:320-4. [Crossref] [PubMed]
- Cobianchi L, Peloso A, Filisetti C, et al. Serum lactate level as a useful predictor of clinical outcome after surgery: an unfulfilled potential? J Thorac Dis 2016;8:E295-7. [Crossref] [PubMed]
- Mallat J, Lemyze M, Tronchon L, et al. Use of venous-to-arterial carbon dioxide pressure difference to guide resuscitation therapy in septic shock. World J Crit Care Med 2016;5:47-56. [Crossref] [PubMed]
- Mekontso-Dessap A, Castelain V, Anguel N, et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med 2002;28:272-7. [Crossref] [PubMed]
- Ospina-Tascón GA, Hernández G, Cecconi M. Understanding the venous-arterial CO2 to arterial-venous O2 content difference ratio. Intensive Care Med 2016;42:1801-4. [Crossref] [PubMed]
- Silbert BI, Litton E, Ho KM. Central venous-to-arterial carbon dioxide gradient as a marker of occult tissue hypoperfusion after major surgery. Anaesth Intensive Care 2015;43:628-34. [Crossref] [PubMed]
- Teboul JL, Mercat A, Lenique F, et al. Value of the venous-arterial PCO2 gradient to reflect the oxygen supply to demand in humans: effects of dobutamine. Crit Care Med 1998;26:1007-10. [Crossref] [PubMed]
- Bakker J, Vincent JL, Gris P, et al. Veno-arterial carbon dioxide gradient in human septic shock. Chest 1992;101:509-15. [Crossref] [PubMed]
- Mallat J, Pepy F, Lemyze M, et al. Central venous-to-arterial carbon dioxide partial pressure difference in early resuscitation from septic shock: a prospective observational study. Eur J Anaesthesiol 2014;31:371-80. [Crossref] [PubMed]
- Morel J, Gergelé L, Dominé A, et al. The venous-arterial difference in CO2 should be interpreted with caution in case of respiratory alkalosis in healthy volunteers. J Clin Monit Comput 2017;31:701-7. [Crossref] [PubMed]
- Ospina-Tascón GA, Umaña M, Bermúdez W, et al. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O 2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med 2015;41:796-805. [Crossref] [PubMed]
- Mallat J, Lemyze M, Meddour M, et al. Ratios of central venous-to-arterial carbon dioxide content or pressure to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care 2016;6:10. [Crossref] [PubMed]
- Mesquida J, Saludes P, Gruartmoner G, et al. Central venous-to-arterial carbon dioxide difference combined with arterial-to-venous oxygen content difference is associated with lactate evolution in the hemodynamic resuscitation process in early septic shock. Crit Care 2015;19:126. [Crossref] [PubMed]
- Monnet X, Julien F, Ait-Hamou N, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med 2013;41:1412-20. [Crossref] [PubMed]
- Vallet B, Pinsky MR, Cecconi M. Resuscitation of patients with septic shock: please «mind the gap»! Intensive Care Med 2013;39:1653-5. [Crossref] [PubMed]
- Tsaousi GG, Karakoulas KA, Amaniti EN, et al. Correlation of central venous-arterial and mixed venous-arterial carbon dioxide pressure gradient with cardiac output during neurosurgical procedures in the sitting position. Eur J Anaesthesiol 2010;27:882-9. [Crossref] [PubMed]
- Futier E, Robin E, Jabaudon M, et al. Central venous O2 saturation and venous-to-arterial CO2 difference as complementary tools for goal-directed therapy during high-risk surgery. Crit Care 2010;14:R193. [Crossref] [PubMed]
- Robin E, Futier E, Pires O, et al. Central venous-to-arterial carbon dioxide difference as a prognostic tool in high-risk surgical patients. Critical Care 2015;19:227. [Crossref] [PubMed]
- Silva JM, Oliveira AM, Segura JL, et al. A large Venous-Arterial PCO(2) Is Associated with Poor Outcomes in Surgical Patients. Anesthesiol Res Pract 2011;2011:759792. [Crossref] [PubMed]
- ESICM LIVES. 2018. Available online: https://icm-experimental.springeropen.com/articles/10.1186/s40635-018-0201-6
- Cavaliere F, Martinelli L, Guarneri S, et al. Arterial-venous PCO2 gradient in early postoperative hours following myocardial revascularization. J Cardiovasc Surg (Torino) 1996;37:499. [PubMed]
- Habicher M, von Heymann C, Spies CD, et al. Central Venous-Arterial pCO2 Difference Identifies Microcirculatory Hypoperfusion in Cardiac Surgical Patients With Normal Central Venous Oxygen Saturation: A Retrospective Analysis. J Cardiothorac Vasc Anesth 2015;29:646-55. [Crossref] [PubMed]
- Morel J, Grand N, Axiotis G, et al. High veno-arterial carbon dioxide gradient is not predictive of worst outcome after an elective cardiac surgery: a retrospective cohort study. J Clin Monit Comput 2016;30:783-9. [Crossref] [PubMed]
- Guinot PG, Badoux L, Bernard E, et al. Central Venous-to-Arterial Carbon Dioxide Partial Pressure Difference in Patients Undergoing Cardiac Surgery is Not Related to Postoperative Outcomes. J Cardiothorac Vasc Anesth 2017;31:1190-6. [Crossref] [PubMed]
- Du W, Long Y, Wang XT, et al. The Use of the Ratio between the Veno-arterial Carbon Dioxide Difference and the Arterial-venous Oxygen Difference to Guide Resuscitation in Cardiac Surgery Patients with Hyperlactatemia and Normal Central Venous Oxygen Saturation. Chin Med J 2015;128:1306-13. [Crossref] [PubMed]
- Abou-Arab O, Braik R, Huette P, et al. The ratios of central venous to arterial carbon dioxide content and pressure to arteriovenous oxygen content are not associated with overall anaerobic metabolism in postoperative cardiac surgery patients. PLoS One 2018;13:e0205950. [Crossref] [PubMed]
- Ruokonen E, Soini HO, Parviainen I, et al. Venoarterial CO2 gradient after cardiac surgery: relation to systemic and regional perfusion and oxygen transport. Shock 1997;8:335-40. [Crossref] [PubMed]
- Van der Linden P, Rausin I, Deltell A, et al. Detection of tissue hypoxia by arteriovenous gradient for PCO2 and pH in anesthetized dogs during progressive hemorrhage. Anesth Analg 1995;80:269-75. [PubMed]
- Kocsi S, Demeter G, Érces D, et al. Central venous-to-arterial CO2-gap may increase in severe isovolemic anemia. PLoS One 2014;9:e105148. [Crossref] [PubMed]
- Dubin A, Ferrara G, Kanoore Edul VS, et al. Venoarterial PCO2-to-arteriovenous oxygen content difference ratio is a poor surrogate for anaerobic metabolism in hemodilution: an experimental study. Ann Intensive Care 2017;7:65. [Crossref] [PubMed]
- Mallat J, Mohammad U, Lemyze M, et al. Acute hyperventilation increases the central venous-to-arterial PCO2 difference in stable septic shock patients. Ann Intensive Care 2017;7:31. [Crossref] [PubMed]
- Morel J, Gergele L, Vereche D, et al. Do fluctuations of PaCO2 impact on the venous-arterial carbon dioxyde gradient? Critical Care 2011;15:456. [Crossref] [PubMed]
- Kara A, Akin S, Ince C. The response of the microcirculation to cardiac surgery. Curr Opin Anaesthesiol 2016;29:85-93. [Crossref] [PubMed]
- De Backer D, Orbegozo Cortes D, Donadello K, et al. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014;5:73-9. [Crossref] [PubMed]
- Edul VSK, Enrico C, Laviolle B, et al. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med 2012;40:1443-8. [Crossref] [PubMed]
- Atasever B, Boer C, Goedhart P, et al. Distinct alterations in sublingual microcirculatory blood flow and hemoglobin oxygenation in on-pump and off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2011;25:784-90. [Crossref] [PubMed]
- Koning NJ, Simon LE, Asfar P, et al. Systemic microvascular shunting through hyperdynamic capillaries after acute physiological disturbances following cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 2014;307:H967-75. [Crossref] [PubMed]
- Bansch P, Flisberg P, Bentzer P. Changes in the sublingual microcirculation during major abdominal surgery and post-operative morbidity. Acta Anaesthesiol Scand 2014;58:89-97. [Crossref] [PubMed]
- Jhanji S, Lee C, Watson D, et al. Microvascular flow and tissue oxygenation after major abdominal surgery: association with post-operative complications. Intensive Care Med 2009;35:671-7. [Crossref] [PubMed]
- Vallet B, Teboul JL, Cain S, et al. Venoarterial CO(2) difference during regional ischemic or hypoxic hypoxia. J Appl Physiol 2000;89:1317-21. [Crossref] [PubMed]



