
Monitoring and optimization of the microcirculation during CPB
Cardiac surgery can be associated with organ dysfunction and the degree of organ dysfunction is associated with length of ICU stay and mortality (1). In a majority of cardiac surgical procedures cardiopulmonary bypass (CPB) is used to facilitate surgery. It has been shown that conventional CPB (cCPB) can be associated with significant decreases in both cerebral blood flow (CBF) and cerebral metabolic rate for oxygen (CMRO2), during laminar flow in comparison with use of a pulsed waveform (2). Pulsatile CPB when compared with cCPB has also been associated with decreased incidences of end organ dysfunction (3). Although there is evidence that high risk patients, particularly those with renal failure, have improved outcomes with pulsatile CPB (4), currently non-pulsatile CPB remains standard of care in a majority of cardiac centers. Until recently it has been difficult to examine tissue microcirculatory perfusion during cardiac surgery and CPB.
CPB is known to induce an inflammatory reaction and release of inflammatory mediators that may exert effects on the microcirculation similar to severe sepsis. De Backer et al. observed alterations in microcirculatory function during and after cardiac surgery in 15 patients using Side Stream Dark Field Imaging (SSDFI) that were present after induction of anesthesia and persisted for 24 hours post-operatively (5). Very few studies have been published describing the microcirculation during and after cardiac surgery using non-invasive monitoring. While the mechanism for decreased organ perfusion with cCPB remains speculative, it is reported that non-pulsatile perfusion during cardiac surgery results in decreased microcirculatory oxygenation in the skin and gut (6).
Near-infrared reflectance spectroscopy (NIRS) uses analysis of absorptance spectra in the near-infrared range (680 to 1,100 nm) to determine regional tissue hemoglobin oxygen saturation (7). Near-infrared light readily penetrates biological tissues and is absorbed mainly by hemoglobin. According to Beer's Law, the near-infrared spectroscopy signal is limited to vessels with a diameter less than 1 mm (arterioles, capillaries, and venules). Commercially available tissue NIRS (tNIRS) devices detect relative percentages of oxygenated and de-oxygenated blood in tissue to determine tissue oxygen saturation. tNIRS has been used to assess oxygen balance in the microcirculation of skeletal muscles of shock patients (8). Since cCPB invokes a significant inflammatory response impairing microcirculatory perfusion (9), tNIRS is increasingly being utilized to assess tissue microcirculation during CPB,
The first studies using tNIRS and vascular occlusion test (VOT) in conjunction with SSDFI demonstrated similar directional changes with both techniques when comparing pulsatile and non-pulsatile perfusion during CPB (10). In a randomized cohort study of 20 high risk patients, O’Neill et al. have recently reported that compared with cCPB, roller pump generated pulsatility is associated with improved microcirculatory blood flow and tissue oxygen saturation (10).
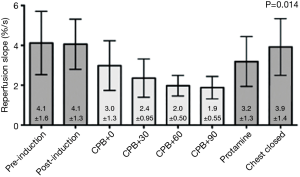
Recent studies using tNIRS have demonstrated a progressive reduction in microcirculatory vasoreactivity during cCPB (11). Using a pneumatic cuff to evoke a quantifiable ischemic challenge, the vascular occlusion test (VOT) uses tNIRS to measure the vasoreactivity of peripheral tissue during and after CPB and cCPB was shown in a small study to induce a progressive reduction in microcirculatory responsivity with longer duration cCPB that returned to baseline after separation from cCPB as shown in Figure 1 (11). This study did raise the concern that the progressive decrease in microvascular vasoreactivity seen during cCPB, if not restored to baseline after separation from CPB, could be a harbinger of end organ dysfunction. Further studies have amplified this concern (12).
In a study of 254 patients undergoing cardiac and thoracic surgery in whom tNIRS and VOT were employed sequentially before and after surgery, although conventional hemodynamic values, such as cardiac output and blood pressure, did not differ between the groups, on multivariable regression and linear analyses low VOT recovery slope on postoperative day 1 was associated with increases of composite complications and hospital length of stay (12).
A more recent study again using tNIRS and VOT during cCPB and pulsatile CPB has shown better preservation of microcirculatory reactivity with pulsatile perfusion (10). As such it is likely that cCPB induces progressive reduction in microcirculatory perfusion—not only as shown in peripheral muscle, but similar phenomena likely also occur in deep tissue and organs and may well contribute to end organ dysfunction associated with cCPB (13). Use of pulsatile CPB has been associated with decreased rates of renal failure (14).
Taken together these studies would indicate that cCPB induces progressive decrease in microcirculatory vasoreactivity and that those patients in whom abnormal VOT—indicative of microcirculatory dysfunction—persists into the postoperative period, have a significantly greater incidence of end-organ dysfunction.
Use of minimally invasive extracorporeal circulation (MiECC) for CPB has been associated with a variety of improved clinical outcomes in comparison to cCPB (15). It is emphasized that MiECC represents not only a low volume circuit technology but rather that it engenders an approach that is focused on minimizing all procedural aspects of cardiac surgery from amount of fluids administered to duration of postoperative ventilation. Various endpoints including a survival benefit and reduced transfusion and higher hemoglobin levels, as well as better preservation of renal function and lowered levels of inflammatory biomarkers have all been associated with MiECC and may well reflect improved microcirculatory perfusion (15).
As such, it is tNIRS and VOT by enabling continuous and non-invasive assessment of tissue microcirculatory perfusion and oxygenation during CPB—when coupled with clinical outcomes—that will further help evaluate and improve circulatory support techniques. The role of MiECC and its impact on microcirculatory flow will be investigated and should provide further evidence of the fundamental role preservation of the microcirculation plays in helping to further improve patient outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Patila T, Kukkonen S, Vento A, et al. Relation of the sequential organ failure assessment score to morbidity and mortality after cardiac surgery. Ann Thorac Surg 2006;82:2072-8. [Crossref] [PubMed]
- Murkin JM, Farrar JK. The influence of pulsatile vs non-pulsatile cardiopulmonary bypass on cerebral blood flow and metabolism. Anesthesiology 1989;71:A41. [Crossref]
- Serraino GF, Marsico R, Musolino G, et al. Pulsatile cardiopulmonary bypass with intra-aortic balloon pump improves organ function and reduces endothelial activation. Circ J 2012;76:1121-9. [Crossref] [PubMed]
- Milano AD, Dodonov M, Van Oeveren W, et al. Pulsatile cardiopulmonary bypass and renal function in elderly patients undergoing aortic valve surgery. Eur J Cardiothorac Surg 2015;47:291-8; discussion 298. [Crossref] [PubMed]
- De Backer D, Dubois MJ, Schmartz D, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. Ann Thorac Surg 2009;88:1396-403. [Crossref] [PubMed]
- Sicsic JC, Duranteau J, Corbineau H, et al. Gastric mucosal oxygen delivery decreases during cardiopulmonary bypass despite constant systemic oxygen delivery. Anesth Analg 1998;86:455-60. [Crossref] [PubMed]
- Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009;103 Suppl 1:i3-13. [Crossref] [PubMed]
- Doerschug KC, Delsing A S, Schmidt G A, et al. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol 2007;293:H1065-71. [Crossref] [PubMed]
- Wan S, Leclerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest 1997;112:676-92. [Crossref] [PubMed]
- O'Neil MP, Alie R, Guo LR, et al. Microvascular Responsiveness to Pulsatile and Nonpulsatile Flow During Cardiopulmonary Bypass. Ann Thorac Surg 2018;105:1745-53. [Crossref] [PubMed]
- Smith RS, Murkin JM. A novel assessment of peripheral tissue microcirculatory vasoreactivity using vascular occlusion testing during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2014;28:1217-20. [Crossref] [PubMed]
- Kim TK, Cho YJ, Min JJ, et al. Microvascular reactivity and clinical outcomes in cardiac surgery. Crit Care 2015;19:316. [Crossref] [PubMed]
- Murkin JM, Martzke JS, Buchan AM, et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. I. Mortality and cardiovascular morbidity. J Thorac Cardiovasc Surg 1995;110:340-8. [Crossref] [PubMed]
- Nam MJ, Lim CH, Kim HJ, et al. A Meta-Analysis of Renal Function After Adult Cardiac Surgery With Pulsatile Perfusion. Artif Organs 2015;39:788-94. [Crossref] [PubMed]
- Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 2016;22:647-62. [Crossref] [PubMed]


