
Primary spontaneous pneumothorax: time for surgery at first episode?
Primary spontaneous pneumothorax (PSP) generally occurs in healthy young people between the ages of 10 and 30 years (1). It remains an important global health problem with an annual incidence of 7.4–18 (age-adjusted incidence) and 1.2–6 cases per 100,000 population, among men and women respectively. The pathogenesis of PSP is still unclear and the presence of blebs/bullae or macro/microscopical pleural modification (e.g., “pleural porosity”) are currently the most accredited causes. Moreover, risk factors for PSP include tall-and-thin body habitus, male gender, and smoking (2). Without intervention, recurrence rates after PSP are variably reported, with studies quoting rates as low as 14% (3) and as high as 49% (4) at 1 year, up to 50% in patients followed-up for 5 years (5,6). According to Swedish cost analysis data, the economic burden of PSP approximates 130 million USD/year with around 20,000 individuals affected per year (7).
While the treatment of choice in case of recurrent or complicated spontaneous pneumothorax (PTX) is clearly established (8-10), the management of patients with PSP at the first episode is still an open debate.
In the first episodes of PSP, simple aspiration or pleural drainage are the current settled first line therapies with an immediate success rate of 59.3% and 68% respectively (11), whereas several studies have been conducted on the advantages and disadvantages of the conservative approach (thoracentesis and pleural drainage) versus surgery.
Analysing advantages and disadvantages of intercostal drainage (ITD) versus thoracentesis, a systematic review and meta-analysis by Kim et al., and a Cochrane systematic review by Carson were conducted in 2017 (7,12). These studies showed that whereas ITD was more effective than simple aspiration in the timely resolution of PTX with higher rates of immediate success, the hospitalization was shorter with aspiration and the recurrence rate within 1 year did not vary between the initial approaches.
As regards the surgical approach, according to a recent systematic review and network meta-analysis of randomized clinical trials, in patients with persistent or recurrent PSP, pleural abrasion (mechanical pleurodesis) or pleurectomy performed by thoracotomy is the most effective treatment to prevent recurrent PTX and video-assisted thoracoscopic surgery (VATS) with talc poudrage (chemical pleurodesis) is related with noteworthy reduction in relapses compared to VATS with mechanical pleurodesis. However, VATS has significantly less complications compared with thoracotomy and is superior to it in terms of length of hospital stay, pain relief and compromised lung function (13).
The two most recent International guidelines, published by The British Thoracic Society in 2010 and The European Respiratory Society in 2015, typically advocate surgical treatment for patients with non-resolving PTX, for a recurrent episode, haemopneumothorax, bilateral pneumothoraces or for occupations at risk. Several retrospective studies have revealed that, in patients with first episode PSP, VATS provide improved outcomes in terms of duration of chest tube, hospital stay and recurrence rates (14). Moreover, several authors have pointed out that young patients would willingly undergo surgery rather than live with the uncertainty of recurrences (15). Though in the future the indication for definitive treatment may enlarge to patients expected to have a high risk of recurrence after an initial episode, there is a lack of randomized clinical trial evidence.
Winnie Hedevang Olesen from Odense, Denmark, led a recently published randomized controlled, multicentre trial at three public university-based cardiothoracic departments (Odense University Hospital, Aarhus University Hospital, Aalborg University Hospital) to assess whether patients may benefit from surgery following their first PSP episode. Eligible patients were young and healthy individuals between 18 and 40 years of age admitted to the hospital within a 7-year period (01/08/2009–04/11/2016). On admittance at either of the hospitals, appointed local investigators oversaw the initial treatment of the first episode of PSP and they ascertained that chest tube drainage was indicated according to the standard of care for acute PTX. Then all patients were screened, the eligible ones were identified and informed by the investigators. Out of 457 patients admitted with PSP, 373 patients met the inclusion criteria, but ultimately only 181 were enrolled. In more detail 59/373 were not willing to take part in the trial; 81/373 eligible patients were inexplicably not requested to participate; 51/373 were excluded because referring to the regional cardiothoracic centre belatedly (>5 days after the episode); 1/373 patient underwent primary surgery without prior computed tomography (CT) because of an incomplete lung re-expansion after ITD and then was excluded. Of the 181 included patients, 151 were men and 30 women (ratio =5.0). According to the study protocol, all enrolled patients underwent multislice 64-row high-resolution computed tomography (HRCT) without contrast (spiral acquisition CT-scan) to discover the presence and actual size of blebs/bullae, that were defined as air-filled lesions <1 and ≥1 cm respectively. Eighty-eight patients showed blebs: 50 were randomly allocated to chest tube treatment and 38 to VATS. Ninety-three patients had bullae: 43 were randomly assigned to chest tube placing and 50 to VATS. Importantly it should be noted, because the authors did not use block randomization, the patients were not allocated equally between the 4 sub-groups.
VATS was performed according to a 3-port technique with a 10-mm inferior camera port and included bullectomy of all visible blebs/bullae, and if none were discovered, resection of the apical part of the upper lobe was executed; a mechanical pleurodesis of all visible parietal pleura was associated.
The analysed variables were recurrence (yes/no), allocated treatment (conventional chest tube treatment or VATS), bulla size (<1 or >1 cm) and referral cardiothoracic centre.
In 12 (13%) patients assigned to the conservative treatment arm, more than one chest tube was placed during their first admission, and in 38 (42%) patients, suction was applied to completely expand the lung. Eight patients crossed over from conservative arm to VATS due to prolonged air leakage even though proper ITD treatment. Three patients crossed from the surgical cohort to conservative one due to medical reasons. Two patients did not undergo surgery due to severe anxiety. The primary end point of the RCT was ipsilateral recurrent spontaneous PTX detected by chest radiography. Secondary end points were complications and length of hospital stay. The interim analysis reported in the paper was performed when the first 150 registered patients had concluded 1-year follow-up given that all four subgroups had at least 50% of the intended inclusions: the median follow-up of all 181 included patients was 59 (range, 9–97) months. Analysing the recurrence rate, 43 (23%) patients experimented ipsilateral recurrence: 32 (34%) patients in conservative treatment arm versus 11 (13%) patients in VATS arm. The multivariate analysis revealed that surgery had a significant impact on overall recurrence comparing with conventional chest tube treatment: 13% vs. 34% respectively (P=0.0012), with no differences between the three-referral cardiothoracic centres (P=0.35). The unadjusted Cox regression analysis stratified by the four subgroups revealed a risk reduction in recurrence in patients with bullae ≥1 cm who underwent primary VATS [hazard ratio (HR) 0.3, P=0.014], but only tendency towards statistical significance in patients with blebs <1 cm, indicating that the presence of large-scale dystrophic lesions may be an independent risk factor for recurrence.
Stratifying by approach, the authors detected a higher recurrence rate in patients with large bullae conservatively treated. The Cox regression analysis adjusted by assigned treatment and bullae size of all conservatively managed patients versus surgically treated, showed that the allocation to conservative treatment was the only significant predictor of recurrence (P=0.0011).
The authors also detected a trend towards larger bullae being at higher risk of recurrence (HR 2.4, P=0.045): conservatively treated patients with bullae ≥2 cm were identified as high-risk population (HR 4.4, P=0.045). The presence of blebs and bullae on CT in predicting recurrence is controversial. Four previous studies have given conflicting results, with two finding no association (3,16) and two finding an association (17,18). A recent meta-analysis found that CT scoring systems could not reliably predict recurrence in PTX study (19). Interestingly, the scoring system with the most encouraging results, the dystrophic severity score (DSS), was not found to significantly predict recurrence in this study.
Length of hospital stay at first admittance was not significantly different (P=0.42) following either treatment: 4.1 days (95% CI: 3.4–4.8) in the surgical group and 3.8 days (95% CI: 3.0–4.3) after ITD management. It should be noted that when time in hospital for patients readmitted for their elective surgery was included, surgical patients had a significantly longer median length of stay of 7.1 days (95% CI: 6.4–7.9, P<0.001).
Surgery was not without complications, with three (3%) surgical patients undergoing reintervention due to bleeding from the intercostal arteries in one of the portholes. No patient related outcomes were reported, although it is stated that this will be published at a later date. Presently it is unclear if the amount of pain experience by the patients were comparable. Earlier observational work of 185 patients with PSP who underwent VATS demonstrated that 27% of patients had pain post-op, with 16.5% having pain for longer than 12 weeks (20).
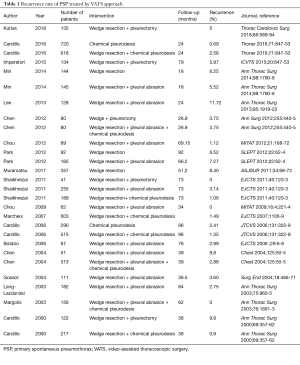
Several aspects of the methodology deserve attention. Totally, 276 of the 457 (60%) of the patients assessed for eligibility were excluded, with 84 not meeting inclusion criteria and 81 omitted for unknown reasons. The protocol stated that patients who presented with small, asymptomatic pneumothoraces were treated with observation only, and it remains unclear how many were excluded for this reason. It has been proposed that the majority of PSPs can be managed with observation, with a recently completed randomised trial examining this issue due to be published shortly (21). Additionally, 51 patients were excluded if the patients were not referred for surgery for more than five days, and one patient was excluded due to inadequate lung re-expansion with initial chest tube drainage. The exclusion of these subjects are possible confounders, particularly as they did not receive a HRCT, and it is unclear whether the positive predictive value related to large bullae on HRCT is applicable to all patients. The paper suggests that surgery could be more cost-effective, despite longer length of stays, due to higher recurrence rates in the conservatively managed cohort. However, this is speculative, as no formal cost-effective analysis was performed, and further work is required. At interim analysis, the results strongly favoured VATS over conservative treatment in all three cardiothoracic centres, and according to the protocol, the Authors decided to stop enrolling patients for ethical reasons. This study showed VATS to be associated with a very low operative risk, surgical trauma, and complication rate with a short in hospital stay and a recurrence rate much lower than the standard treatment (ITD). Interestingly the rate of recurrence showed in the Danish RCT (13%) was higher than that reported in the literature (Table 1). In a paper reporting VATS talc poudrage in 1,415 patients with PSP, we showed a recurrence rate of 1.9% (5), other studies have reported a recurrence rate around 3–4% (Table 1) which compared to the high recurrence rate after drainage only (17–54%) (4,6), could give much more authority to the approach proposed by the present paper.
Full table
Olesen and his Danish co-workers should be commended for an excellent work, performing an RCT in a difficult study population, which has improved our understanding. Whilst these results are very encouraging, it must be recognised that the majority of patients with PSP will not develop a recurrence, with a recent meta-analysis of studies of medically managed patients (both first and subsequent presentation) demonstrating a recurrence rate of 32% (19). This study shows that with a number need to treat (NNT) of 4.8, five patients will have to undergo surgical procedure to avoid one recurrence. This information, together with the unpublished patient-related outcomes of pains and anxiety levels should help clinicians and patients make an informed decision.
In conclusion, the RCT by Olesen and co-workers demonstrates decreased recurrence rates with surgery at first occurrence of PSP (NNT 4.8) and identifies CT criteria, that could recognise which patients will benefit most. We eagerly await their publication of quality of life outcomes, which should help inform patient choice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Noppen M, De Keukeleire T. Pneumothorax. Respiration 2008;76:121-7. [Crossref] [PubMed]
- Kepka S, Dalphin JC, Parmentier AL, et al. Primary Spontaneous Pneumothorax Admitted in Emergency Unit: Does First Episode Differ from Recurrence? A Cross-Sectional Study. Can Respir J 2017;2017:2729548. [Crossref] [PubMed]
- Ouanes-Besbes L, Golli M, Knani J, et al. Prediction of recurrent spontaneous pneumothorax: CT scan findings versus management features. Respir Med 2007;101:230-6. [Crossref] [PubMed]
- Chen JS, Chan WK, Tsai KT, et al. Simple aspiration and drainage and intrapleural minocycline pleurodesis versus simple aspiration and drainage for the initial treatment of primary spontaneous pneumothorax: an open-label, parallel-group, prospective, randomised, controlled trial. Lancet 2013;381:1277-82. [Crossref] [PubMed]
- Cardillo G, Bintcliffe OJ, Carleo F, et al. Primary spontaneous pneumothorax: a cohort study of VATS with talc poudrage. Thorax 2016;71:847-53. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Carson-Chahhoud KV, Wakai A, van Agteren JE, et al. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev 2017;9:CD004479. [PubMed]
- Cardillo G, Carleo F, Giunti R, et al. Videothoracoscopic talc poudrage in primary spontaneous pneumothorax: a single-institution experience in 861 cases. J Thorac Cardiovasc Surg 2006;131:322-8. [Crossref] [PubMed]
- Cardillo G, Facciolo F, Giunti R, et al. Videothoracoscopic treatment of primary spontaneous pneumothorax: a 6-year experience. Ann Thorac Surg 2000;69:357-61. [Crossref] [PubMed]
- Cardillo G, Facciolo F, Regal M, et al. Recurrences following videothoracoscopic treatment of primary spontaneous pneumothorax: the role of redo-videothoracoscopy. Eur J Cardiothorac Surg 2001;19:396-9. [Crossref] [PubMed]
- Ayed AK, Chandrasekaran C, Sukumar M. Aspiration versus tube drainage in primary spontaneous pneumothorax: a randomised study. Eur Respir J 2006;27:477-82. [Crossref] [PubMed]
- Kim MJ, Park I, Park JM, et al. Systematic review and meta-analysis of initial management of pneumothorax in adults: Intercostal tube drainage versus other invasive methods. PLoS One 2017;12:e0178802. [Crossref] [PubMed]
- Vuong NL, Elshafay A, Thao LP, et al. Efficacy of treatments in primary spontaneous pneumothorax: A systematic review and network meta-analysis of randomized clinical trials. Respir Med 2018;137:152-66. [Crossref] [PubMed]
- Chambers A, Scarci M. In patients with first-episode primary spontaneous pneumothorax is video-assisted thoracoscopic surgery superior to tube thoracostomy alone in terms of time to resolution of pneumothorax and incidence of recurrence? Interact Cardiovasc Thorac Surg 2009;9:1003-8. [Crossref] [PubMed]
- Morimoto T, Fukui T, Koyama H, et al. Optimal strategy for the first episode of primary spontaneous pneumothorax in young men. J Gen Intern Med 2002;17:193-202. [Crossref] [PubMed]
- Martínez-Ramos D, Angel-Yepes V, Escrig-Sos J, et al. Usefulness of computed tomography in determining risk of recurrence after a first episode of primary spontaneous pneumothorax: Therapeutic implications. Arch Bronconeumol 2007;43:304-8. [Crossref] [PubMed]
- Primavesi F, Jager T, Meissnitzer T, et al. First Episode of Spontaneous Pneumothorax: CT-based Scoring to Select Patients for Early Surgery. World J Surg 2016;40:1112-20. [Crossref] [PubMed]
- Casali C, Stefani A, Ligabue G, et al. Role of blebs and bullae detected by high-resolution computed tomography and recurrent spontaneous pneumothorax. Ann Thorac Surg 2013;95:249-55. [Crossref] [PubMed]
- Walker SP, Bibby AC, Halford P, et al. Recurrence rates in primary spontaneous pneumothorax: a systematic review and meta-analysis. Eur Respir J 2018;52:1800864. [Crossref] [PubMed]
- Herrmann D, Klapdor B, Ewig S, et al. Initial management of primary spontaneous pneumothorax with video-assisted thoracoscopic surgery: a 10-year experience. Eur J Cardiothorac Surg 2016;49:854-9. [Crossref] [PubMed]
- Brown SG, Ball EL, Perrin K, et al. Study protocol for a randomised controlled trial of invasive versus conservative management of primary spontaneous pneumothorax. BMJ Open 2016;6:e011826. [Crossref] [PubMed]


