
Risk factors for outcomes of acute respiratory distress syndrome patients: a retrospective study
Introduction
Acute respiratory distress syndrome (ARDS) is a common pulmonary disorder in critically ill patients in intensive care unit (ICU), first proposed in 1994 by the American-European Consensus Conference (AECC) (1). Following this the Berlin Definition was developed in 2012, which categorizes ARDS as mild, moderate or severe based on degree of hypoxemia (2). ARDS can be caused by many reasons, such as trauma, transfusion history, infection, sepsis, pneumonia and even ventilator-induced lung injury (3-5). The morbidity of ARDS is high, which remains a challenging problem in ICU. The famous LUNG SAFE study demonstrated that hospital mortality from ARDS was 40.0%, and ARDS appeared to be under-recognized and under-treated (6). Despite optimal conventional therapies, mortality rate in different studies varies from 30% to 70%, with an average mortality of 50% (7,8), which significantly affects outcomes of patients in ICU.
Previous studies have shown that some indicators correlate closely with the outcomes of ARDS patients. It was reported that ICU and hospital survival decreased when ARDS severity increased (6). In a multivariate analysis done by Monchi et al., the severity of the underlying medical conditions, oxygenation index, mechanism of lung injury, cirrhosis, and occurrence of right ventricular dysfunction were all independent risk factors for increased death of ARDS patients (9). In ARDS patients, an optimal compliance by protective mechanical ventilation with optimal positive end-expiratory pressure (PEEP) application was associated with less organ dysfunction and lower mortality (10). Several studies have revealed that ventilation variables such as driving pressure can stratify risk of death in ARDS patients (11-13). In addition, pulmonary infection (14), ventilator-associated pneumonia (VAP) (15), transfusion (16), fluid overload (17), organ failure (18), acute physiology and chronic health evaluation II (APACHE II) score and lactate level (19) were reported to be related to ARDS patients’ outcomes. In general, these parameters are usually related to the degree of pulmonary function impairment and non-pulmonary organ failures. It has also been shown that certain inflammatory factors, such as interleukin (IL)-1β, IL-6 and IL-18 are consistent and efficient predictors for ARDS patients (20,21). However, those parameters cannot be detected immediately at bedside. This prolongs the diagnostic progress. Few studies have focused on the clinically accessible parameters related to the outcomes of patients, and tools to evaluate outcomes are limited. It remains challenging to identify patients who are at highest risk of poor outcome.
Therefore, screening of the clinically accessible risk factors most relevant to the outcomes of ARDS patients are of high importance in clinic. In this study, the possible accessible parameters were selected and used to stratify the risk for outcomes including patient survival and duration of mechanical ventilation. This may provide a comprehensive basis for the clinical assessment of prognosis and guidance for future treatment of ARDS patients.
Methods
Study design and patient population
A total of 209 ARDS patients admitted to the general ICU in the Second Affiliated Hospital of Harbin Medical University from Jan 1st, 2016 to Jan 1st, 2017 were included in this study. Data were retrospectively recorded if patients fulfilled the inclusion criteria based on Berlin Definition of ARDS (2). All ARDS patients were divided to be mild, moderate or severe based on following criteria: mild (200 mmHg < PaO2/FiO2 ≤300 mmHg), moderate (100 mmHg < PaO2/FiO2 ≤200 mmHg), and severe (PaO2/FiO2 ≤100 mmHg). Patients who were less than 18 years old were excluded, as were patients who died within 24 hours of admission to the ICU. The study was approved by the Ethics Committee of our hospital.
Data collection
Clinical data of ARDS patients were collected from the medical record system of our hospital. The selection of risk factors was based on previous studies (6,9-19), including the following: baseline demographic information (age and gender); underlying disease (diabetes mellitus, hypertension, chronic heart failure, chronic liver disease or chronic renal disease); APACHE II score; severity of ARDS (mild, moderate or severe); etiology of ARDS (direct ARDS such as pneumonia or aspiration, indirect ARDS such as non-pulmonary sepsis, pancreatitis); infection site (pulmonary or extrapulmonary); presence of VAP; transfusion history; the number of organ failure (heart failure, liver failure, renal failure, hematologic failure or other organ dysfunction); hemodynamic parameters including heart rate (HR), mean arterial pressure (MAP) and central venous pressure (CVP); ventilator parameters including respiratory rate (RR), tidal volume, plateau pressure (Pplat), driving pressure (i.e., Pplat-PEEP), lung compliance and PEEP; blood gas parameters including pH, PaO2/FiO2, lactate level and base excess (BE); laboratory parameters including white blood cell (WBC) count, absolute neutrophil count (ANC), neutrophil percentage, hemoglobin (Hb) level, platelet count, serum albumin and creatinine level; urine output (UO).
The primary outcome indicator was patient survival. The duration of mechanical ventilation, ICU length of stay (LOS) and hospital LOS were also recorded.
Statistical analysis
All statistical analyses were performed using Statistical Analysis System (SAS) (version 9.1.3, SAS Institute Inc., Cary, NC, USA). Data which are normally distributed were presented as mean ± standard deviation (SD), comparisons between two groups were carried out with two-sample t-test; data which are not normally distributed were presented as median and interquartile range median (IQR), comparisons between two groups were performed with Wilcoxon rank-sum test (The statistic for Wilcoxon rank-sum test is Z) and comparisons among multiple groups were carried out with the Kruskal-Wallis H test. Categorical variables were described as frequency and percentage, and comparisons were completed with χ2 test or Fisher’s exact test. Spearman’s rank correlation coefficient was used to analyze the correlation of duration of mechanical ventilation with ICU LOS and hospital LOS, and the correlation of lactate level with pH and BE, respectively. Logistic regression analysis was used to analyze the influence factors for survival and duration of mechanical ventilation (3 levels). P<0.05 was considered as statistically significant.
Results
General characteristics of the study population
During the study period, 1,113 patients were admitted to the ICU (962 survivors and 151 non-survivors), the in-hospital mortality of the whole ICU population was 13.57% (151/1,113). Among 209 patients who met the Berlin Definition, 2 patients were found to be admitted for twice. Therefore, 207 patients were eventually included in the study, including 85 patients who survived to discharge or be transferred to other wards, 41 patients who abandoned treatment, and 81 non-survivors who deceased in hospital. This mortality 39.13% (81/207) was higher in ARDS population. As a result, 166 patients (41 patients who abandoned treatment were excluded) were included in the comparisons and logistic regression analysis. The period prevalence of mild, moderate and severe ARDS was 39.61% (82/207), 37.20% (77/207) and 23.19% (48/207), respectively.
Comparisons of mortality in ARDS patients
The comparisons of mortality rates were shown in Table 1, which revealed significant differences in 12 factors (APACHE II score, severity of ARDS, infection site, number of organ failure, MAP, plateau pressure, driving pressure, lung compliance, pH, PaO2/FiO2, lactate level and BE) (P<0.05), suggesting patients with higher APACHE II score, higher severity of ARDS, pulmonary infection, more organ failures, lower MAP, higher plateau pressure, higher driving pressure, lower lung compliance, lower pH, lower PaO2/FiO2, higher lactate level and lower BE had higher mortality rates.
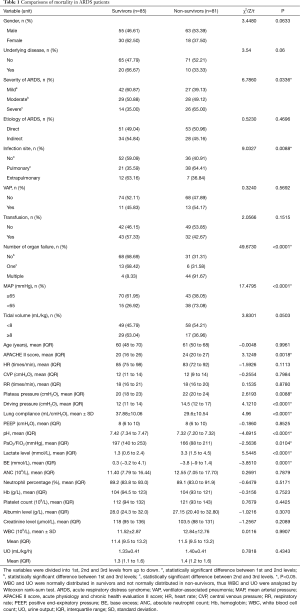
Full table
Risk factors for death in ARDS patients
Multivariate logistic regression was carried out to screen the influence factors for death (12 independent factors as shown in Table 2). The independent variables were manually selected, including the 12 significant factors from above comparison results and 2 control variables (age and gender). However, lactate level was negatively correlated with pH and BE (rs=−0.73478, P<0.0001; rs=−0.47773, P<0.0001) respectively, so only lactate level was included in the model to avoid multicollinearity. Three variable selection methods (forward, backward and stepwise) all showed that APACHE II score (OR 3.4316; 95% CI: 1.3130–8.9686; P=0.0119), number of organ failure (OR 3.4928; 95% CI: 1.9775–6.1693; P<0.0001), MAP (OR 5.1049; 95% CI: 1.8317–14.2274; P=0.0018), driving pressure (OR 6.0017; 95% CI: 2.1746–16.5641; P=0.0005) and lactate level (OR 4.0754; 95% CI: 1.6114–10.3068; P=0.0030) were influence factors for survival, suggesting that the ARDS patients with higher APACHE II score, more organ failures, lower MAP, higher driving pressure and higher lactate level had higher risk of death (shown in Table 3).

Full table
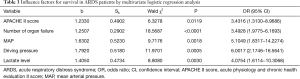
Full table
Comparisons of duration of mechanical ventilation in ARDS patients
The Spearman correlation analysis showed that the duration of mechanical ventilation was positively correlated to ICU LOS and hospital LOS respectively (rs=0.78426, P<0.0001; rs=0.39558; P<0.0001). Considering that the duration of mechanical ventilation was most relevant to ARDS, and ICU LOS and hospital LOS can be affected by many other factors, we focused on duration of mechanical ventilation in the following analysis. Among 166 patients, 164 ones who received mechanical ventilation were included in the comparisons. As shown in Table 4, significant differences in 7 factors (VAP, transfusion history, number of organ failure, MAP, pH, lactate level and BE) (P<0.05) were observed, suggesting that the patients with VAP, transfusion history, one organ failure, lower MAP, higher pH, lower lactate level, and higher BE required prolonged duration of mechanical ventilation. In addition, the duration of mechanical ventilation of non-survivors was shorter than survivors.
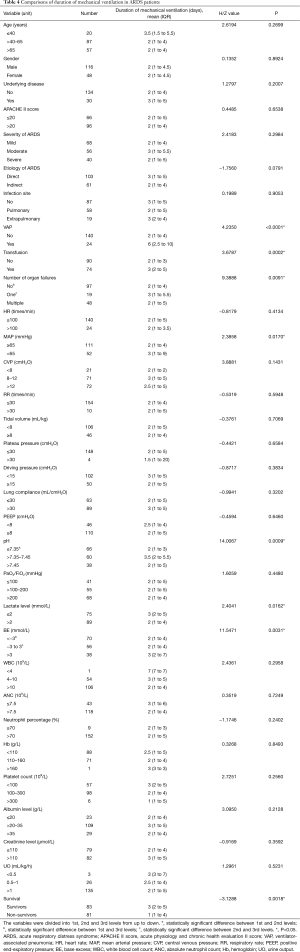
Full table
Risk factors for prolonged duration of mechanical ventilation in ARDS patients
Multivariate logistic regression was used to screen the influence factors (9 independent factors as shown in Table 5) for duration of mechanical ventilation (divided into 3 levels based on percentile). The independent variables were manually selected, including 7 significant factors from comparison results (VAP, transfusion history, number of organ failure, MAP, pH, lactate level, and BE) and 4 control variables (age, gender, APACHE II score and severity of ARDS). However, lactate level was negatively correlated with pH and BE (rs=−0.73478, P<0.0001; rs=−0.47773, P<0.0001) respectively, so only lactate level was included in the model to avoid multicollinearity. Three variable selection methods (forward, backward and stepwise) all showed that severity of ARDS (OR 1.6715; 95% CI: 1.0307–2.7108; P=0.0373), VAP (OR 7.3746; 95% CI: 2.9799–18.2505; P<0.0001) and transfusion history (OR 2.2822; 95% CI: 1.0462–4.9783; P=0.0381) were influence factors for duration of mechanical ventilation, suggesting that the ARDS patients with higher severity of ARDS, VAP and transfusion history had higher risk of prolonged mechanical ventilation, as shown in Table 6.
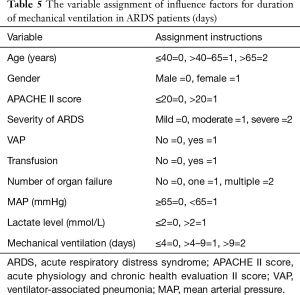
Full table

Full table
Discussion
ARDS is a life-threatening condition affecting the survival of ICU patients (22). In the past decade, short-term mortality has been significantly reduced. However, long-term outcomes are still unsatisfactory despite the widespread application of lung-protective ventilation and better general supportive therapy (23). Given the fact that some parameters cannot be immediately obtained at bedside, clinically accessible parameters may help ICU specialists better understand the risk for the poor outcomes of patients.
In this current study, the total in-hospital mortality of ARDS patients was higher than the whole ICU population. The mortality of ARDS patients in this study was quite high, which was similar to the mortality in other studies (24). Airway driving pressure was the only ventilation variable related to the survival and has received considerable attention after a previous research in which Amato et al. reported that patients with a driving pressure (i.e., Pplat-PEEP) of more than 14 cmH2O on day 1 showed a worse outcome (13). The principle is that driving pressure was the surrogate for cyclic lung strain which was most easily accessible and easiest to calculate, and amplitude of cyclic stretch is more closely related to cell and tissue damage other than the maximal level of stretch (25). LUNG SAFE study showed that higher driving pressure was associated with increased risk of death (6). Following this, many other studies further verified to support this view (11,12).
Other than driving pressure, the influence factors for survival include APACHE II score, number of organ failure, MAP, driving pressure and lactate level, which are related to other pathological processes but not directly to lung. It has been reported that the APACHE II score applied with arterial blood lactate clearance rate has significance in assessing the prognosis of ARDS patients (19). Not surprisingly, APACHE II score >20 is a risk factor for ARDS patients. Complicating organ failures such as acute kidney injury, liver failure, heart failure and even MODS may significantly aggravate patients’ outcomes (26). Multiple organ failure has been previously reported as the major cause of mortality among ARDS patients (27,28). In our study, the risk of death increased in patients with more organ failures, which further verified the previous study.
ARDS is frequently associated with hemodynamic instability. The instability is also the main factor which affects outcomes. Factors that lead to shock in ARDS patients include pulmonary hypertension, detrimental effects of mechanical ventilation on the right ventricle, as well as sepsis (29). Although other profound hemodynamic indicators may better suggest severity, MAP is the most rapid, convenient one and a key hemodynamic parameter that represents the hemodynamic stability and perfusion of the body. The presence of MAP <65 mmHg indicates cardiovascular failure and therefore it is closely related to survival.
Plasma lactate concentration may be useful as a metabolic marker of tissue hypoxia, which has been applied in diagnosis, treatment and prognosis. Lung is the main lactate-producing organ in ARDS patients. High lactate level is characteristic of metabolic acidosis, a condition known to be predictive of acute lung injury in severely traumatized patients (30). Recent studies showed that metabolic acidosis is associated with high mortality in critically ill patients (31,32), which is consistent with our result.
Of particular importance is the fact that a large number of patients with prolonged mechanical ventilation may have increased risk of infection and higher hospitalization cost, as well as experience decreased life quality (33), so the duration of mechanical ventilation was selected as secondary outcome. The factors that affected the duration of mechanical ventilation are more related to the lung itself while the risk factors for survival are more systematic. Severity of ARDS represents the severity of pulmonary impairment, thus more severe ARDS needs a prolonged ventilation time. The presence of VAP may complicate the outcome of ARDS. The VAP associated morbidity and mortality rates are high but whether VAP is associated with ARDS patients’ outcomes still remains a matter of debate (34,35). In our study, VAP can be used as a risk factor for prolonged mechanical ventilation but not survival. In ARDS, it is widely recommended to maintain the hemoglobin level with packed red blood cells (PRBC) transfusions when tissue oxygenation and perfusion cannot improve oxygen delivery (36). However, more evidences have indicated that transfusion induces adverse effects such as lung injury in critically ill patients, and further increases the modality of MODS (37-39). These factors may all prolong the duration of mechanical ventilation.
Besides, several unexpected results were also observed. It is widely accepted that severity of ARDS classified as mild, moderate, and severe based on PaO2/FiO2 is positively correlated with survival (2,6,40). APACHE II score and severity of ARDS are both key parameters closely related to the severity of patients, so age, gender, APACHE II score and severity of ARDS were included as control variables in logistic regression analysis. In our study, although the comparisons showed that mortality rate was different when severity of ARDS differs, multivariate logistic regression analysis showed that it was not an influence factor for survival. Recent reports have demonstrated that stratification of severity of ARDS did not completely correlate with mortality (41,42). The condition of ARDS patients in ICU is usually complicated, while the classification of ARDS severity is only based on lung related factors. It has been shown that other complicating conditions may also affect the survival of patients (43). Furthermore, another study reported that APACHE/SOFA scores are positively related to increased ARDS severity (44). Also, the severity was only determined at onset of ARDS, which might change with the development of disease and affect the survival. Thus, factors which are more systematic such as APACHE II score were strongly related to patients’ survival. Infection was also not identified as a risk factor for survival in logistic regression analysis. However, in comparison between pulmonary and extrapulmonary infection in ARDS patients, the mortality of patients with pulmonary infection was higher than extrapulmonary infection, which was also supported by a previous study (14). Surprisingly in comparisons of duration of mechanical ventilation, patients with lower lactate level, higher pH, higher BE and one organ failure (compared with multiple organ failure) required prolonged duration of mechanical ventilation. The possible reason is that patients with higher lactate level, lower pH, lower BE and multiple organ failure had higher mortality rate, and the duration of mechanical ventilation of non-survivors was shorter than survivors.
There are also some limitations in this study. First, this is a retrospective study, the conclusion should be verified by certain prospective study in the future. Secondly, this study was performed in a single-center, the population size in our study is comparatively small and ARDS patients are sometimes under-treated. It will be our goal to conduct a multi-center study to further verify our conclusion in the future. Thirdly, the parameters we investigated can be interfered after initiation of treatment, most of the parameters were collected at the onset of ARDS. We are sometimes unable to identify the exact onset of ARDS. In future study, it will be helpful to develop a tool for predicting outcomes based on dynamic parameters during the development of ARDS.
Conclusions
The mortality of ARDS is higher than the overall mortality of the whole population in ICU. Higher APACHE II score, more organ failures, lower MAP, higher driving pressure and higher lactate level are the risk factors for survival. Higher severity of ARDS, VAP and transfusion history are risk factors for prolonged duration of mechanical ventilation. From these factors, intensivists may benefit from better understanding of the disease course and patient outcomes, thus enabling a more efficient therapeutic regimen for ICU patients.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (No. 81571871 and No. 81770276) and Nn10 program of Harbin Medical University Cancer Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University (IRB number: KY2019-150), and written informed consent was waived.
References
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Janz DR, Ware LB. Biomarkers of ALI/ARDS: pathogenesis, discovery, and relevance to clinical trials. Semin Respir Crit Care Med 2013;34:537-48. [Crossref] [PubMed]
- Janz DR, Ware LB. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J Intensive Care 2015;3:20. [Crossref] [PubMed]
- Lium B. Adult respiratory distress syndrome (ARDS). Incidence, clinical findings, pathomorphology and pathogenesis. A review. Nord Vet Med 1983;35:38-47. [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Aisiku IP, Yamal JM, Doshi P, et al. The incidence of ARDS and associated mortality in severe TBI using the Berlin definition. J Trauma Acute Care Surg 2016;80:308-12. [Crossref] [PubMed]
- Moss M. Mortality is the only relevant outcome in ARDS: yes. Intensive Care Med 2015;41:141-3. [Crossref] [PubMed]
- Monchi M, Bellenfant F, Cariou A, et al. Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med 1998;158:1076-81. [Crossref] [PubMed]
- Pintado MC, de Pablo R, Trascasa M, et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care 2013;58:1416-23. [Crossref] [PubMed]
- Guerin C, Papazian L, Reignier J, et al. Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care 2016;20:384. [Crossref] [PubMed]
- Tojo K, Yoshida T, Yazawa T, et al. Driving-pressure-independent protective effects of open lung approach against experimental acute respiratory distress syndrome. Crit Care 2018;22:228. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Sheu CC, Gong MN, Zhai R, et al. The influence of infection sites on development and mortality of ARDS. Intensive Care Med 2010;36:963-70. [Crossref] [PubMed]
- Timsit JF, Zahar JR, Chevret S. Attributable mortality of ventilator-associated pneumonia. Curr Opin Crit Care 2011;17:464-71. [Crossref] [PubMed]
- Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med 2005;33:1191-8. [Crossref] [PubMed]
- Gattinoni L, Cressoni M, Brazzi L. Fluids in ARDS: from onset through recovery. Curr Opin Crit Care 2014;20:373-7. [Crossref] [PubMed]
- Flaatten H, Gjerde S, Guttormsen AB, et al. Outcome after acute respiratory failure is more dependent on dysfunction in other vital organs than on the severity of the respiratory failure. Crit Care 2003;7:R72. [Crossref] [PubMed]
- Wu WH, Niu YY, Zhang CR, et al. Combined APACH II score and arterial blood lactate clearance rate to predict the prognosis of ARDS patients. Asian Pac J Trop Med 2012;5:656-60. [Crossref] [PubMed]
- Makabe H, Kojika M, Takahashi G, et al. Interleukin-18 levels reflect the long-term prognosis of acute lung injury and acute respiratory distress syndrome. J Anesth 2012;26:658-63. [Crossref] [PubMed]
- Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995;107:1062-73. [Crossref] [PubMed]
- Raymondos K, Dirks T, Quintel M, et al. Outcome of acute respiratory distress syndrome in university and non-university hospitals in Germany. Crit Care 2017;21:122. [Crossref] [PubMed]
- Chiumello D, Coppola S, Froio S, et al. What's Next After ARDS: Long-Term Outcomes. Respir Care 2016;61:689-99. [Crossref] [PubMed]
- Siddiqui S, Puthucheary Z, Phua J, et al. National survey of outcomes and practices in acute respiratory distress syndrome in Singapore. PLoS One 2017;12:e0179343. [Crossref] [PubMed]
- Protti A, Andreis DT, Monti M, et al. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med 2013;41:1046-55. [Crossref] [PubMed]
- Bellomo R, Ronco C, Mehta RL, et al. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care 2017;7:49. [Crossref] [PubMed]
- Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest 2005;128:525-32. [Crossref] [PubMed]
- Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med 2013;39:275-81. [Crossref] [PubMed]
- Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts' opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42:739-49. [Crossref] [PubMed]
- Eberhard LW, Morabito DJ, Matthay MA, et al. Initial severity of metabolic acidosis predicts the development of acute lung injury in severely traumatized patients. Crit Care Med 2000;28:125-31. [Crossref] [PubMed]
- Hu J, Wang Y, Geng X, et al. Metabolic acidosis as a risk factor for the development of acute kidney injury and hospital mortality. Exp Ther Med 2017;13:2362-74. [Crossref] [PubMed]
- Silva GB Junior, Daher Ede F, Mota RM, et al. Risk factors for death among critically ill patients with acute renal failure. Sao Paulo Med J 2006;124:257-63. [Crossref] [PubMed]
- Wang ZY, Li T, Wang CT, et al. Assessment of 1-year Outcomes in Survivors of Severe Acute Respiratory Distress Syndrome Receiving Extracorporeal Membrane Oxygenation or Mechanical Ventilation: A Prospective Observational Study. Chin Med J (Engl) 2017;130:1161-8. [Crossref] [PubMed]
- Kobayashi H, Uchino S, Takinami M, et al. The Impact of Ventilator-Associated Events in Critically Ill Subjects With Prolonged Mechanical Ventilation. Respir Care 2017;62:1379-86. [Crossref] [PubMed]
- Forel JM, Voillet F, Pulina D, et al. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care 2012;16:R65. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Crossref] [PubMed]
- Gauvin F, Spinella PC, Lacroix J, et al. Association between length of storage of transfused red blood cells and multiple organ dysfunction syndrome in pediatric intensive care patients. Transfusion 2010;50:1902-13. [Crossref] [PubMed]
- Karam O, Tucci M, Bateman ST, et al. Association between length of storage of red blood cell units and outcome of critically ill children: a prospective observational study. Crit Care 2010;14:R57. [Crossref] [PubMed]
- Rajasekaran S, Sanfilippo D, Shoemaker A, et al. Respiratory Impairment after Early Red Cell Transfusion in Pediatric Patients with ALI/ARDS. Crit Care Res Pract 2012;2012:646473. [Crossref] [PubMed]
- DesPrez K, McNeil JB, Wang C, et al. Oxygenation Saturation Index Predicts Clinical Outcomes in ARDS. Chest 2017;152:1151-8. [Crossref] [PubMed]
- Hernu R, Wallet F, Thiolliere F, et al. An attempt to validate the modification of the American-European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med 2013;39:2161-70. [Crossref] [PubMed]
- Caser EB, Zandonade E, Pereira E, et al. Impact of distinct definitions of acute lung injury on its incidence and outcomes in Brazilian ICUs: prospective evaluation of 7,133 patients. Crit Care Med 2014;42:574-82. [Crossref] [PubMed]
- Killien EY, Mills B, Watson RS, et al. Risk Factors on Hospital Arrival for Acute Respiratory Distress Syndrome Following Pediatric Trauma. Crit Care Med 2018;46:e1088-96. [Crossref] [PubMed]
- George T, Viswanathan S, Karnam AH, et al. Etiology and Outcomes of ARDS in a Rural-Urban Fringe Hospital of South India. Crit Care Res Pract 2014;2014:181593. [Crossref] [PubMed]


