Myelomatous pleural effusion as an initial sign of multiple myeloma—a case report and review of literature
Introduction
Multiple myeloma (MM) is one of the most common and represents 10% of all the malignant hematological diseases which mainly affects bone marrow although extramedullary tissues may be infiltrated as well. Pleural effusion may be a sign of thoracic involvement affecting about 6% of patients with MM (1), It is particularly rare (<1%) for MM patients to present myelomatous pleural effusion, especially for those with pleural effusion as an initial sign (2). Only 16 cases reported in English literature since 2000. We describe a case of MM presented initially as pleural effusion that was diagnosed and treated in our hospital, and reviewed the current literature on clinical manifestation, laboratory examination, diagnosis, treatment and prognosis.
Case report
A 53-year-old male presented with a 6-month history of dry cough, mild fever and night sweat. Two months prior to the admission, he was diagnosed as “Tuberculous pleuritis is possible” in the local clinic, and was given triple antituberculous treatment (isoniazide, rifampin and ethambutal) for 2 months. But his conditions did not change evidently. There was no chest pain, hemoptysis or palpitation. He was a chronic smoker for over 30 packs per years. Physical examinations only showed decreased breath sound, sporadic rhonchi and moist rale in the bilateral lower hemithorax. He preferred in sitting position. The patient’s past history, social history, family history, and review of system were otherwise unremarkable.
Blood investigations revealed the following values: white blood cell (WBC) count: 2.5×109/L (40.7% neutrophils, 46.2% lymphocytes, 8.5% monocytes, 1.7% basophils and 0.0% eosinophils); hemoglobin, 76 mg/L; platelet count, 137×109/L; total protein, 93.1 g/L; albumin, 27.2 g/L; globulin, 65.9 g/L; blood calcium, 1.82 mmol/L (2.25-2.75 mmol/L), uric acid, 454.0 µmol/L; erythrocyte sedimentation rate (ESR), 43 mm/h; carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) are in normal range; thrombin time (TT), 24.3 s; D-dimer, 0.55 ng/L; T-SPOT.TB was negative. The serum light chain kappa: 4,210 mg/dL; the serum light chain lamda: 58.4 mg/dL. The serum immunoglobulin A: 351.00 mg/L, M: 153.00 mg/L, G: 52.7 g/L.
The pleural fluid was light yellow and highly cellular, in which 75% was mononuclear cells. Results from the analyses of the right side pleural fluid indicate an exudative type according to the Light criteria (3), which contained total protein 56.2 g/L, albumin 18.3 g/L, globulin 37.9 g/L, A/G 0.5, lactic dehydrogenase (LDH) 142.0 U/L, a-hydroxybutyrate dehydrogenase (aHBDH) 172.7 U/L, adenosine deaminase (ADA) 62.4 U/L. No acid-resistant bacilli were found. Computed tomography (CT) image of the chest (axial view) indicated bilateral sided pleural effusion and distinctive pleural nodular-like thickening as shown in Figure 1. The posteroanterior skull radiographs demonstrated low craniofacial bones density, and saccate transparent area could be seen without any signs of fractures. The posteroanterior view of pelvis was normal. The single photon emission computed tomography (SP-ECT) scan indicated metabolic disturbance of skull bones and elevated metabolism condition of the middle of left humerus.

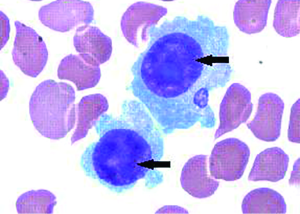
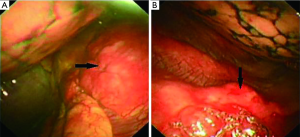
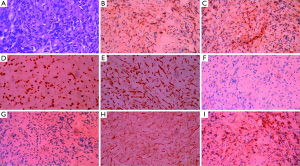
The bone marrow aspiration biopsy showed that hyperplasia of original plasma cells (1.5%) and active hyperplasia of naïve plasma cells (15.5%) (Figure 2). A thoracoscopic pleural biopsy of right side through video-assisted thoracic surgery was performed. Multiple nodules of pleural surface and partial lung collapse could be seen (Figure 3). The pathology of the specimen revealed abnormal proliferation of plasmocytes on hematoxylin and eosin (HE) stains. The immunohistochemistry test of the specimen showed: CD31 (+), CD34 (+), Ki67 (50%+), CD138 (+), CD38 (+), Kappa (+), Lambda (–), MUM1 (+) (Figure 4).
The patient was finally diagnosed as MM with pleural infiltration, IgG-k type, stage II [ISS criteria (4)] based on the clinical manifestations, physical and laboratory examinations, radiographic findings, pathological and immunohistochemistry results. The patient was transferred accordingly to the Hematology Department for chemotherapy (bortezomib, dexamethasone and thalidomide) immediately. After two cycle of chemotherapy, the β-2 microglobulin dropped from 5.4 to 3.2 mg/L, the serum globulin dropped from 65.6 to 19.1 g/L. Chest CT showed that the bilateral sided pleural effusion was gone completely (Figure 5). The overall condition of the patient is good and currently under routine follow-ups.

Review criteria
We conducted a detailed search of the literatures in English published between 2000 and 2013 in MEDLINE and PubMed using search criteria [(“pleural effusion” and “MM”) or “myelomatous pleural effusions”]. The search led to 64 case reports of MM related pleural effusion including those from the relevant references. We read either the full texts or the abstracts thoroughly and finally decided to include 16 cases about MM of patients with the inclusion criteria: (I) the patients with MM presenting initially with pleural effusion; and (II) the patients are proved to have myelomatous pleural effusion by cytological biopsy of pleural effusion or pleural biopsy. We retrospectively reviewed these patients’ data, including general information, laboratory indexes, diagnostic methods, managements, outcome and etc.
Results
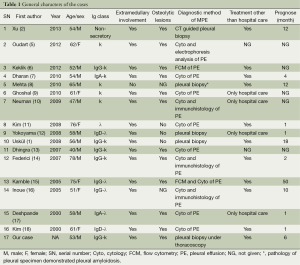
The overall schematic general information, classification of MM, and clinical manifestations are shown in Table 1. Among the 17 cases in this review (including our case report), the mean age is 58.9-year-old (ranging from 40- to 76-year-old) with different gender distribution and the ratio of men to women is nearly 2:1.
Full table
In this series, IgG is the most common type of MM (7/17) in contrast to the IgA predominance in previous reports (19,20). The vast majority of patients presented with dyspnea (14/17) and the other symptoms include cough (4/17), chest pain (3/17), fatigue (3/17), back pain (2/17), mild fever (2/17), and expectoration (1/17). All of these symptoms are nonspecific and mainly caused by massive pleural effusion.
Some important laboratory indexes of MM including the analysis of pleural effusion, blood and bone marrow are shown in Table 2. Among the pleural effusions, eight were left-sided, five were right-sided, and four were bilateral sided and all of them were proved to be exudative. Four of the 17 cases had serosanguinous pleural fluid. Biochemical tests of pleural effusion were performed in ten patients.

Full table
Elevated ADA levels were proved in four patients (>70 IU/L), and elevated LDH levels were found in four patients. However, we did not find any evidence of pulmonary tuberculosis in the relevant literatures (see the detailed discussion). Some important laboratory evidence, such as anemia (hemoglobin <12 g/dL) was seen in 6 of the 17 patients and the serum beta-2 microglobulin and albumin were only both given in three case reports (Table 2). According to ISS criteria (4) and based on the available information, only three patients can be categorized as MM and all of them were at stage II.
Some medullary and extramedullary involvement proofs, diagnostic method, treatment and prognosis are also shown in Table 1. Six of the 17 patients were involved in nodular-like thickening of pleural, three cases had rib lyric lesions and two patients had spinal lyric lesions combined with mediastinal abnormalities. The overwhelming majority had osteolytic lesions (12/15) proved by either X-ray or SP-ECT and at the time of diagnosis, only one of the patients showed more than 80% of plasmacytosis in the bone marrow.
Nearly all of the patients (16/17) had associated pleural or chest wall plasmacytomas, and the final diagnosis of myelomatous pleural effusion was established by pleural biopsy in 4 patients, pleural cytology in 11 patients, immunohistochemistry method in 3 patients, flow cytometry (FCM) in 2 patients, and 1 patient’s pleural fluid was originated from pleural amyloidosis.
Among all the reported case, the treatments described in this series of literature were quite complicated and most of the patients received chemotherapy (11/15) with two of them received radiotherapy after chemotherapy, and only one patient received blood stem cell transfusion. However, the positive response rate was less than 50% and the overall median survival time was 4 months (ranging from 3 to 50 months).
Discussion
MM is one of the most common and represents 10% of all the malignant hematological diseases which mainly affects bone marrow although extramedullary tissues may be infiltrated as well. Pleural effusion may be a sign of thoracic involvement affecting about 6% of patients with MM (1). Oudart and his colleagues (5) had summarized the six etiologic factors which lead to pleural effusion in MM, including congestive heart failure secondary to amyloidosis, chronic renal failure, nephritic syndrome secondary to renal tubular infiltration with paraprotein and development of glomerular damage, direct infiltration of pleural fluid from adjacent tissues, hypoalbuminemia, pulmonary embolism, secondary neoplasm, lymphatic drainage obstruction by tumor infiltration, infection and pleural myelomatous involvement. Of all these six factors listed above, myelomatous involvements of pleural and adjacent tissues were the most common one which brought about pleural exudates in our series.
About 80 reports about myelomatous pleural effusion have been reported so far and most of the symptoms were seen in a late stage of MM with a poor prognosis of the median survival time hardly exceeding 4 months (21). According to previous reports, left-sided pleural effusion is mostly seen (22). However, bilateral sided pleural effusion caused by pleural myelomatous is extremely rare and only three cases have been reported so far (2,13,23).
One noteworthy finding in our series is the high frequency of light chain kappa type of myeloma (24%) and high level in serum or pleural effusion. In the 2008 WHO classification, the light chain type usually less than 20% (24). In these light chain kappa subtype of myeloma patients, the k/λ ratios in the pleural effusion were all higher than that found in the serum, indicating a possible local synthesis of k light chain. Oudart et al. had proposed the ratio difference may be a reflection of variant clearance mechanism in the pleural fluid and blood (5).
Another noteworthy finding is the high ADA activities in pleural effusion. In our series, four cases were found with elevated ADA activities. High level of ADA activities in the pleural fluid strongly recommends tuberculous pleural effusion, and the sensitivity and specificity can reach 92% and 90%, respectively (25). The reported cutoff values of ADA activities to exclude tuberculous pleural effusion ranged from 40 to 60 IU/L (26-31). However, the elevated ADA activity had also been reported in other diseases, such as breast cancer, non-Hodgkin’s lymphoma, and some malignant hematologic diseases (32-35). The ADA activities of the four patients reviewed in this paper exceeded the upper limit of the cutoff value. Since ADA is an enzyme expressed in activated T-lymphocytes, the elevation of ADA activity in the pleural effusion can be used as an indicator of active local inflammatory response (36,37). Therefore, since the ADA activity may indicate an activation or alteration of the immune system, and this also can be used to explain why most of the patients with elevated ADA activity in myelomatous pleural effusion also had enlarged lymph nodes, especially mediastinal lymph nodes. However, this hypothesis needs to be tested with more researches in the future.
The biochemistry tests of blood and fluid are important for giving good diagnostic orientations. The high quantitative of globulins, low levels of albumin and high levels of calcium suggest the possibilities of MM. When we consider about it, the blood and fluid investigation such as electrophoresis and immunofixation electrophoresis should be given to the patients. However, for those patients with pleural effusion as one of the first signs, mostly they visit respiratory department at first. Sometimes, their routine biochemistry tests of blood and fluid are non-specific. In this case, it is hard for a respiratory physician to take hematological malignancy into consideration and need to do some traumatic investigation to give final diagnosis.
Cytological identification of malignant plasma cells within the pleural effusion has been considered as the best diagnose method of myelomatous pleural effusion (38). However, due to the limited number of malignant plasma cells and potential in vitro degeneration, it may fail to make diagnosis. Since 4 of the 17 patients who were misdiagnosed as tuberculousis pleurisy and subsequently proven to be myelomatous pleural effusion by pleural biopsy, this indicates that pleural biopsy may also be the most efficient and reliable method in differentiating myelomatous and tuberculosis pleural effusion. It had been recommended that in patients with only pleural fluid appearance on CT scan and in those who may have benign pleural effusion, the primary method of diagnosis should be medical thoracoscopy (21). Pleural infiltration of plasma cells is patchy sometimes and it is hard to find abnormal signs of pleural in CT images or B-ultrasonography, and it is unlikely to get positive result in CT/B-ultrasound guided pleural biopsy. However, with the development of thoracoscope technology, pleural biopsy under video-assisted thoracoscope is now not only a safe procedure but also could improve the diagnosis rate with both diagnostic sensitivity and specificity around 100% in pleural effusion from unknown origin (39,40). To our best knowledge, the case reported here is the first one whose pleural infiltration of MM was detected by pleural biopsy under video-assisted thoracoscope.
The overall median survival time for myelomatous pleural effusion is 4 months (ranging from 3 to 50 months), which is much less than 29 months, the median survival time of stage III MM (21). In our series, the treatment methods varied, the response rate was low, and survive time was short. Kamble et al. (15) had concluded that system chemotherapy combined with chest tube drainage or pleurodesis was an excellent palliation in most patients. However, there appears to be no advantage of radiotherapy, blood stem cell transfusion or other aggressive therapy. Therefore, newer drugs and palliation methods need to be developed in the future to prolong survival time and improve the quality of life for these patients.
Due to (I) the limited number of myelomatous pleural effusion cases as an initial clinical manifestation and (II) incomplete information from each individual cases, such as laboratory indexes of serum and pleural effusion, it is challenging at the present stage to establish the precise relationship between test indexes and prognosis of this disease. This not only prevented us from conducting evidence-based medicine but also limited our ability to provide any meaningful prognosis of this particular disease. This also indicates that further research in myelomatous pleural effusion is warranted.
Conclusions
Our review shows that myelomatous pleural effusion is rare. Its clinical and laboratory findings are non-specific. Definitive diagnosis relies on the histopathology of pleural biopsy or pleural effusion. Thoracoscopic pleural biopsy is reliable, safe and effective. Chemotherapy is the mainstay of treatment for myelomatous pleural effusion. However, the response rate is low with an overall median survival time of 4 months.
Acknowledgements
The author would like to thank Professor Dianzheng Zhang for the English language review.
Disclosure: The authors declare no conflict of interest.
References
- Uskül BT, Türker H, Emre Turan F, et al. Pleural effusion as the first sign of multiple myeloma. Tuberk Toraks 2008;56:439-42. [PubMed]
- Xu XL, Shen YH, Shen Q, et al. A case of bilateral pleural effusion as the first sign of multiple myeloma. Eur J Med Res 2013;18:7. [PubMed]
- Light RW. The Light criteria: the beginning and why they are useful 40 years later. Clin Chest Med 2013;34:21-6. [PubMed]
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23:3412-20. [PubMed]
- Oudart JB, Maquart FX, Semouma O, et al. Pleural effusion in a patient with multiple myeloma. Clin Chem 2012;58:672-4. [PubMed]
- Keklik M, Sivgin S, Pala C, et al. Flow cytometry method as a diagnostic tool for pleural fluid involvement in a patient with multiple myeloma. Mediterr J Hematol Infect Dis 2012;4:e2012063. [PubMed]
- Dharan M. Unilateral pleural effusion as a presenting manifestation of plasma cell myeloma (multiple myeloma): a case report. Acta Cytol 2010;54:780-2. [PubMed]
- Mehta AA, Venkatakrishnan R, Jose W, et al. Multiple myeloma presenting as eosinophilic pleural effusion. Asia Pac J Clin Oncol 2010;6:256-9. [PubMed]
- Ghoshal AG, Sarkar S, Majumder A, et al. Unilateral massive pleural effusion: a presentation of unsuspected multiple myeloma. Indian J Hematol Blood Transfus 2010;26:62-4. [PubMed]
- Neuman G, Denekamp Y. Dyspnea and pleural effusion as presenting clinical manifestations of multiple myeloma. Isr Med Assoc J 2009;11:118-9. [PubMed]
- Kim YJ, Kim SJ, Min K, et al. Multiple myeloma with myelomatous pleural effusion: a case report and review of the literature. Acta Haematol 2008;120:108-11. [PubMed]
- Yokoyama T, Tanaka A, Kato S, et al. Multiple myeloma presenting initially with pleural effusion and a unique paraspinal tumor in the thorax. Intern Med 2008;47:1917-20. [PubMed]
- Dhingra KK, Singhal N, Nigam S, et al. Unsuspected multiples myeloma presenting as bilateral pleural effusion - a cytological diagnosis. Cytojournal 2007;4:17. [PubMed]
- Federici L, Blondet C, Andrès E. Aggressive form of multiple myeloma presenting with specific pleural effusion, neutrophilia, and eosinophilia. Eur J Intern Med 2007;18:348-9. [PubMed]
- Kamble R, Wilson CS, Fassas A, et al. Malignant pleural effusion of multiple myeloma: prognostic factors and outcome. Leuk Lymphoma 2005;46:1137-42. [PubMed]
- Inoue Y, Chua K, McClure RF, et al. Multiple myeloma presenting initially as a solitary pleural effusion later complicated by malignant plasmacytic ascites. Leuk Res 2005;29:715-8. [PubMed]
- Deshpande AH, Munshi MM. Pleural effusion as an initial manifestation of multiple myeloma. Acta Cytol 2000;44:103-4. [PubMed]
- Kim YM, Lee KK, Oh HS, et al. Myelomatous effusion with poor response to chemotherapy. J Korean Med Sci 2000;15:243-6. [PubMed]
- Rodríguez JN, Pereira A, Martínez JC, et al. Pleural effusion in multiple myeloma. Chest 1994;105:622-4. [PubMed]
- Cho YU, Chi HS, Park CJ, et al. Myelomatous pleural effusion: a case series in a single institution and literature review. Korean J Lab Med 2011;31:225-30. [PubMed]
- Kamble R, Wilson CS, Fassas A, et al. Malignant pleural effusion of multiple myeloma: prognostic factors and outcome. Leuk Lymphoma 2005;46:1137-42. [PubMed]
- Kintzer JS Jr, Rosenow EC 3rd, Kyle RA. Thoracic and pulmonary abnormalities in multiple myeloma. A review of 958 cases. Arch Intern Med 1978;138:727-30. [PubMed]
- Makino S, Yamahara S, Nagake Y, et al. Bence-Jones myeloma with pleural effusion: response to alpha-interferon and combined chemotherapy. Intern Med 1992;31:617-21. [PubMed]
- Sabattini E, Bacci F, Sagramoso C, et al. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 2010;102:83-7. [PubMed]
- Liang QL, Shi HZ, Wang K, et al. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med 2008;102:744-54. [PubMed]
- Light RW, Erozan YS, Ball WC Jr. Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med 1973;132:854-60. [PubMed]
- Valdés L, Alvarez D, San José E, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med 1998;158:2017-21. [PubMed]
- Valdés L, Alvarez D, San José E, et al. Value of adenosine deaminase in the diagnosis of tuberculous pleural effusions in young patients in a region of high prevalence of tuberculosis. Thorax 1995;50:600-3. [PubMed]
- Villena V, Navarro-Gonzálvez JA, García-Benayas C, et al. Rapid automated determination of adenosine deaminase and lysozyme for differentiating tuberculous and nontuberculous pleural effusions. Clin Chem 1996;42:218-21. [PubMed]
- Burgess LJ, Maritz FJ, Le Roux I, et al. Use of adenosine deaminase as a diagnostic tool for tuberculous pleurisy. Thorax 1995;50:672-4. [PubMed]
- Ungerer JP, Oosthuizen HM, Retief JH, et al. Significance of adenosine deaminase activity and its isoenzymes in tuberculous effusions. Chest 1994;106:33-7. [PubMed]
- Aghaei M, Karami-Tehrani F, Salami S, et al. Adenosine deaminase activity in the serum and malignant tumors of breast cancer: the assessment of isoenzyme ADA1 and ADA2 activities. Clin Biochem 2005;38:887-91. [PubMed]
- Buyukberber M, Sevinc A, Cagliyan CE, et al. Non-Hodgkin lymphoma with high adenosine deaminase levels mimicking peritoneal tuberculosis: an unusual presentation. Leuk Lymphoma 2006;47:565-8. [PubMed]
- Meier J, Coleman MS, Hutton JJ. Adenosine deaminase activity in peripheral blood cells of patients with haematological malignancies. Br J Cancer 1976;33:312-9. [PubMed]
- Ohata M, Masuda I, Nonaka K, et al. Combination assay of IAP and ADA in hematologic malignancies. Rinsho Byori 1990;38:703-10. [PubMed]
- Liu YC, Shin-Jung Lee S, Chen YS, et al. Differential diagnosis of tuberculous and malignant pleurisy using pleural fluid adenosine deaminase and interferon gamma in Taiwan. J Microbiol Immunol Infect 2011;44:88-94. [PubMed]
- Light RW. Update on tuberculous pleural effusion. Respirology 2010;15:451-8. [PubMed]
- Safa AM, Van Ordstrand HS. Pleural effusion due to multiple myeloma. Chest 1973;64:246-8. [PubMed]
- Ng TH, How SH, Kuan YC, et al. Medical thoracoscopy: Pahang experience. Med J Malaysia 2008;63:298-301. [PubMed]
- Metintas M, Ak G, Dundar E, et al. Medical thoracoscopy vs CT scan-guided Abrams pleural needle biopsy for diagnosis of patients with pleural effusions: a randomized, controlled trial. Chest 2010;137:1362-8. [PubMed]


