
Endoscopic resection for early esophageal carcinoma
Introduction
In 1990 Inoue, a surgeon from Japan, presented the first endoscopic cap resection technique for early neoplastic lesions in the esophagus (1). It took more than a decade to be introduced in the Western world, and to become the standard of care nowadays.
Over the last 15 years a frame shift in treatment options has occurred; from surgery to endoscopic treatment for patients with early esophageal neoplasia. The basic principle for endoscopic treatment is based on the fact that the risk of lymph node metastasis is very small when neoplasia is confined to the mucosal layer of the esophagus. When invading in the submucosa the risk of lymph node metastasis gradually increases since lymph vessels and the larger blood vessels are located in the lower submucosa. The mucosa and submucosal can be both safely resected, as long as the muscle layer is not perforated. Studies have shown that endoscopic treatment is safe in experienced hands with low complications rates and excellent long-term survival (84%) (2-4). Endoscopic resection (ER) preserves the esophagus and significantly reduces the morbidity and mortality risk associated with surgical esophageal resection.
Adenocarcinoma is the predominant esophageal cancer in the western world, with Barrett’s esophagus as the most important risk factor for esophageal adenocarcinoma. In Asia, squamous cell cancer is the most predominant esophageal type of cancer.
Barrett esophagus is the metaplasia of columnar lined epithelium that replaced the normal squamous epithelium, which is secondary to chronic esophageal reflux disease. Patients with Barrett’s esophagus have an increased risk of developing adenocarcinoma with an annual incidence of 0.3–0.5% (5,6).
International guidelines advise endoscopic surveillance in Barrett’s patients with regular intervals to detect neoplastic lesions in early stage (7). Patients who are followed in a surveillance program have shown a lower disease stage and hence a better prognosis. As dysplasia in Barrett’s is difficult to recognize and a general endoscopist in The Netherlands finds an average of one patient with dysplasia per year, it is impossible to develop expertise from a single practice. Therefore, it is advised in the European Guideline that patient with high-grade dysplasia (HGD) and carcinoma should be referred to a center with expertise in this field (7).
Squamous cell carcinoma has a more aggressive behavior than adenocarcinoma, and the risk of lymph node metastasis according to depth of invasion is higher. The risk factors for developing squamous cell dysplasia are: genetic, tobacco and alcohol use (8,9). Endoscopic screening in patients with an increased risk for squamous cell carcinoma, may improve early detection of these lesions. Screening programs are therefore enrolled in endemic areas (e.g., China) or in patients with head and neck cancer (10). Squamous cell cancer arises from the stratified squamous epithelial lining of the organ and develops along an identical dysplastic pathway from low-grade intraepithelial neoplasia to high-grade intraepithelial neoplasia to early squamous cell cancer. Early squamous cell carcinoma is difficult to detect due to its flat appearance, usually a flat Paris type IIa, IIb or IIc lesion. Staining with Lugol (chromoendoscopy) improves visualization of such lesions, especially in moderate and severe dysplasia (11,12). Detection of squamous cell carcinoma in an early stage can allow ER and cure without the need of invasive treatment with chemoradiotherapy or esophagectomy.
Endoscopic evaluation of early esophageal neoplasia
ER techniques are used to remove visible mucosal abnormalities. Visible lesions within the esophagus are described according to the Paris classification (13). In general, flat lesions are subdivided into slightly elevated (0–IIa), completely flat (0–IIb) and slightly depressed (0–IIc) and can be safely resected. Some protruded type 0–Is (sessile) and 0–Ip (polypoid) lesions can be safely resected as well. Deeply ulcerated (Paris type III lesions), are a contraindication for ER.
Endoscopic ultrasound (EUS) is considered the most accurate tool for TNM staging of advanced esophageal cancer, but its role in early Barrett’s neoplasia is debatable. In a retrospective study, EUS did not alter management in patients with non-nodular HGD or mucosal cancer. For patients with nodular neoplasia, resection of the nodule with histologic examination had greater accuracy than staging by EUS (14). Another study demonstrated that when EUS assessment was classified as suspicious for invasion in 84% of them had no evidence of invasion in final pathology. Thus, the assessment of depth of invasion of Barrett’s neoplasia based solely on EUS findings potentially leads to overstaging (15). EUS has, therefore, no clinical impact on the workup of early esophageal carcinoma and should not be used to differentiate between a mucosal and submucosal lesion. In addition, given the very low risk of metastasis in early esophageal carcinoma there is no role for preemptive staging for lymph nodes with EUS or for distant metastasis with CT/PET (16,17). Histological examination of the resection specimen is the best first staging modality.
In case of early squamous cell carcinoma, the vascular pattern of the intrapapillary capillary loop (IPCL), which are the IPCLs of the mucosa as can be seen by using high definition (HD) endoscopy and zoom, can be classified and predict the depth of infiltration (18,19).
Risk of lymph node metastasis
The basic principle for endoscopic treatment of early adenocarcinoma is based on the fact that the chance of lymph node metastasis is very small when neoplasia is confined to the mucosal layer of the esophagus. When invading the submucosa, the risk of lymph node metastasis gradually increases by varying depths of invasion since lymph vessels and the larger blood vessels are located in the lower submucosa.
For adenocarcinoma, ER of mucosal (T1m1-3) and superficial submucosal (T1sm1) lesions can be curatively resected with extremely low risk of lymph node metastasis. Indications for ER of squamous cell carcinoma differ from adenocarcinoma due to the significant higher risk of lymph node metastasis according to the invasion depth. ER of squamous cell carcinoma is an appropriate option in case of low- and high-grade intraepithelial neoplasia and superficial mucosal cancer (T1m1-m2) (20). The risk of lymph node metastasis in T1m3 and T1b squamous cell cancer is significant higher (11.8% versus 24.0–42.8% respectively) compared to adenocarcinoma and therefore only indicated in selected cases (20).
Pathological staging
Resected specimens are pinned down on cork or paraffin blocks to orientate for optimal tissue examination. Histological evaluation of the resected specimen provides the most accurate information on tumor differentiation, lymphovascular infiltration, the depth of invasion, the horizontal margin in endoscopic mucosal resection (EMR). In endoscopic submucosal dissection (ESD) specimen the lateral margins can also be accurately assessed. Histopathological analysis needs to be done by gastrointestinal (GI) pathologists with special interest in GI pathology recognized as such by his/her peers given the significant interobserver variability amongst pathologists (7). Pathological diagnosis indicates the chance of remission and/or the long-term prognosis. The need for adjuvant surgical therapy for the individual patient can be established. In case of unfavorable histological outcome, the individual plan for the patient with or without comorbidities can be balanced by the expected risk of lymph node metastasis versus the peri-operative risk for esophageal resection.
In summary, inspection and evaluation of all mucosal and submucosal lesions need to be done carefully before ER. The risk of lymph node metastasis increases with the depth of invasion. ER of mucosal (T1m1-3) and superficial submucosal (T1sm1) adenocarcinoma can be curative as well as for superficial mucosal (T1m1-m2) squamous cell carcinoma.
ER techniques
ER techniques consist of two main types: EMR and ESD. ESD can be used for en-bloc resection or larger lesions because of the continuous dissection along the submucosal plane. ER is a very precise organ sparing endoscopic surgical technique. Having experience in ER is mandatory to perform these kinds of procedures safely (21).
Principles of EMR
The abnormal mucosa that has to be removed is first delineated visually with the aid of a HD endoscope (Figure 1). Delineation of the lesion is than followed by placing coagulation markers 2 to 5 mm outside the lesion in non-dysplastic mucosa. The markers are placed with the tip of a snare or with Argon plasma coagulation (APC), to be convinced that the correct part of the mucosa is being removed. The abnormal mucosa can be removed en-bloc or by multiple pieces. In a piece meal resection, the lateral margins of the removed lesion cannot be assed. In case of residual dysplastic Barrett, a re-EMR is feasible. A stepwise radical ER of all Barrett’s metaplasia can lead to more severe and bothersome stenosis, but can be an option to be performed in patients who are not responding to ablative therapies (22).
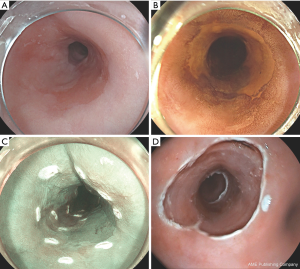
EMR can be performed with the use of the original Inoue cap, containing a rim in which a snare can be placed (manufactured by Olympus). With the ER cap, the mucosa is than lifted with a submucosal injection of saline 0.9% and, when adequately lifted, sucked into the cap and snared. The types of lifting defined by Kato et al. are very useful in predicting the depth of infiltration and resectability (23). More recently caps are used with pre-loaded rubber bands named the multi-band mucosectomy (MBM) technique (Duette® Cook Medical, Ireland or Captivator® Boston-Scientific Ltd.). These MBM ligature kits consistent of a cap and rubber bands, which can be mounted on top of an endoscope.
By using the MBM technique the lesion is sucked into the cap and a rubber band is fired. Afterwards the created pseudopolyp can be snared, preferably below the rubber band. The use of lifting fluids is not necessary because when inflating after firing the rubber band, this band does not hold the propria muscle layer (muscularis propria) which pops out, and the mucosa can be safely resected. In a study comparing the ER cap with the MBM technique the authors conclude that piecemeal ER with MBM is faster and cheaper than with ER-cap (24). ER cap specimens were a little larger, but the depths of the resections were not different. Without submucosal lifting, MBM appeared not to be associated with more perforations (25). The Captivator, a newer band ligator device also consist a cap on the top of the endoscope but the rubber bands are placed more proximal on the endoscope which provides initially a better view trough the cap. The passage of accessories through the scope can be somewhat easier (26). The Captivator resected specimen had a larger surface area and depth in a small retrospective pilot study (27).
Principles of ESD
ESD is a technique to remove lesions by dissecting the submucosal plane (Figure 2). The abnormal mucosa is first delineated by coagulation markers. With this technique, larger lesions can be resected en bloc and this offers more information about complete oncologic resection. ESD is much more technically demanding, time consuming and harbors a greater risk of severe adverse events, such as perforations (28). ESD is reported to be a safe and feasible treatment of complex Barrett’s neoplasia (29). Paris type I lesions are considered a good indication because they often are too large to fit in an ER cap. In Asia, ESD is performed on large scale for removal both types esophageal cancer, especially early squamous cell cancer (30,31).
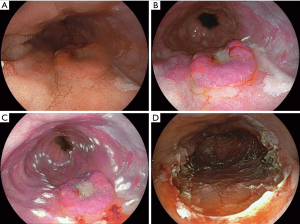
In a recent randomized trial comparing EMR versus ESD of early Barrett’s neoplasia, the authors concluded that in the need for: surgery, neoplasia remission and recurrence, ESD and EMR are both highly effective for ER of early Barrett’s neoplasia (28). ESD achieved a higher R0 resection rate, but for most Barrett’s esophagus patients this has little clinical relevance.
In case of squamous cell carcinoma multiple studies showed a higher curative resection rate of ESD than EMR (32,33) and ESD is evenly effective and safe in elderly patients versus non-elderly patients (9). Thereby, ESD en-bloc resection is preferable in early squamous cell carcinoma.
In conclusion EMR and ESD are both very effective techniques in removing dysplastic mucosa or early cancer. In Paris type I lesions in Barrett’s and for early squamous cell carcinoma ESD is the preferred option.
Management of complications of ERs
ERs can be associated with the following complications: acute intra-procedural bleedings (0.9–24%) (30,34,35) and perforations (1.8–6.9%) (3,30,34). As late complication stricture formation (3.6–49.7%) (3,30,34,35) and delayed bleedings (1.5%) (25) are reported. In large series (1,000 consecutive patients) the overall major complication rate was 1.5%; all complications could be managed conservatively (36).
Acute bleedings are managed during the procedure with the use of snare coagulation or with a coagulating forceps. In case of more severe bleeding, they can be isolated and compressed by the rim of the cap and then treated adequately. Adrenalin can be locally administered through an endoscopic needle. Use of clips is not the preferred treatment of choice because they can hamper further endoscopic treatment. Management of post procedural bleeding during repeated endoscopy is considered similar to any other acute upper GI bleeding.
The most prevalent late complication is esophageal stricture formation (25). Strictures of the esophagus after endoscopic therapy are reported in 3–40% (34) and are usually adequately treated by one or more simple boogie dilatations (3).
Acute perforation can be managed endoscopically by closing the leak by using clips, clips and endoloop combined, or by placing a stent. In case of acute perforation, antibiotics have to be administered. In rare cases immediate esophageal resection is mandatory. In expert centers perforation rates of EMR are less than 1% (25).
In summary, the risk of severe adverse events is low. Most adverse events are managed endoscopically and can be treated conservatively.
Ablative therapies
Several indications for Barrett’s ablation exist: the presence of residual Barrett’s epithelium following ER and Barrett’s segment with pathologically confirmed HGD and/or low-grade dysplasia (LGD) in the absence of visible lesions.
Residual Barrett after ER
Leaving a remnant Barrett after ER causes a risk for metachronous lesions of about 30% (36). The rationale behind this is the field defect within the Barrett with genetic predisposition to develop cancer, the so-called Barrett progressors. Ablation should be performed for eradication of the remaining Barrett’s esophagus. In case of a pop-up lesion with dysplasia or early cancer during ablation therapy of follow-up post-ablation, ER of such lesion is still a good option.
Endoscopic radiofrequency ablation (RFA) is the most widely used ablation technique for Barrett’s epithelium and highly effective to achieve full remission of dysplasia (over 92%) and intestinal metaplasia (over 90%) in large prospective studies (37,38). RFA uses controlled energy to deliver heat for cellular destruction of the superficial mucosal esophageal tissue. There are a number of different devices for local or circumferential RFA, but ablation can only be used on flat mucosa and is not effective in elevated lesions. After RFA the dysplastic Barrett’s will regenerate with neo-squamous epithelium thereby decrease the rate of neoplastic progression (39).
Barrett with HGD or LGD without a visible lesion
Several studies document the risk of progression from LGD to HGD to early adenocarcinoma (40,41). In case of a flat dysplastic lesion (LGD and HGD) in a Barrett’s esophagus (confirmed by an expert pathologist), direct RFA has become standard of care (42,43).
Complications of RFA are pain after the procedure, stricture formation and rarely bleeding. The pain is generally mild and only lasting a few days. Stricture formation is usually easy to treat by bougie dilatation. Delayed bleedings are rare.
Photodynamic therapy (PDT) is a treatment with photosensitizing drug administered in the circulation and activated with light of a specific wavelength that destroy the tumor cells. PDT for eradicating Barrett’s esophagus has been abandoned because of ineffectively and practicality (44,45).
A new ablation technique for eradication of Barrett’s epithelium is cryoablation, potential benefits if this technique may be a deeper ablation with less pain and a lower rate of stricture formation than RFA (46). A recent study compared efficacy in eradicating Barrett’s esophagus and tolerability between balloon-based focal cryoablation with RFA. Results conclude no difference in efficacy after a single treatment and patients reported less pain (47).
Hybrid APC is a combination of a submucosal lifting technique and high energy APC of the mucosa. The fluid cushion reduces the coagulation depth compared to standard APC (48). Manner et al showed that hybrid APC is safe and effectively for ablation of Barrett’s esophagus in centers with expertise and the reported rate of stricture formation is only 2% (49). Terminal ablation by APC compared to surveillance strategy demonstrated to prevent reduction in neoplasia recurrence in follow-up of 2 years (50).
In the recent BRIDE study, APC with RFA in the treatment of remnant Barrett after ER of HGD or early adenocarcinoma was compared (51). The safety and efficacy were similar, but the total costs were substantially higher for RFA. However, the ablation efficacy in this study was only 55.8% whereas large multicenter European studies reported 88–90% efficacy rate (37,38).
Ablation of squamous cell carcinoma
RFA as single therapy for squamous cell carcinoma is not preferable since RFA does not allow histological examination for invasion depth of the squamous cell carcinoma. In that condition, the risk of lymph node metastasis is undetermined, which poses a risk to the patient. Therefore, in flat squamous cell carcinoma tissue resection modalities are still preferred. In a study on RFA of squamous cell dysplasia, a complete remission after RFA in 97% of patients at 12 months without neoplastic progression was seen (52). In a study combining RFA with EMR or ESD for early squamous cell carcinoma 50% complete remission was achieved after additional RFA at 12 months. However, a 30% progression rate to invasive cancer at 1 year was found (53).
Overall, the role of RFA in the treatment armamentarium in squamous cell carcinoma of the esophagus has still to be determined.
Future perspectives
The risk of lymph node metastasis associated with submucosal early adenocarcinoma is mainly based on surgical series (54).
The findings of recent studies which included patients treated with ER indicate that the prevalence of lymph node metastasis in submucosal tumor is lower than in the previously reported surgical series (55-57). At this moment research is continued to explore endoscopic follow-up after ER of a submucosal adenocarcinoma (T1bN0M0) instead of referring patients for additional treatment. These studies will further define the indications for ER of early Barrett’s cancer.
An interesting topic for future work is a combination of endoscopic therapy with isolated thoracolaparoscopy lymphadenectomy without esophagectomy in submucosal esophageal carcinoma. A pilot study performed on the feasibility of sentinel node navigation surgery in combination with thoracolaparoscopic lymphadenectomy without concomitant esophagectomy in early esophageal adenocarcinoma. This hybrid technique seems feasible in patients with high-risk submucosal early esophageal adenocarcinoma (58). More evidence is however needed before applying this technique in clinical practice.
Previous research has shown comparable overall survival between definitive chemoradiotherapy and esophagectomy in early squamous cell carcinoma. However, a higher rate of local recurrence and metachronous lesions was detected in the definitive chemoradiotherapy group during follow-up (59,60). This can be an expected risk factor, because the esophagus in those patients is still in place. An important finding in later research is that combination of ESD followed by chemoradiotherapy instead of chemoradiotherapy alone improved the local control rate in T1aM3 and T1b squamous cell carcinoma (61). A recent Japanese study evaluated the combined treatment of ESD followed by chemoradiotherapy or ESD followed by esophagectomy in clinical T1b esophageal carcinoma and the risk of recurrence (62). ESD followed by chemoradiotherapy was reported as safe and effective for locoregional control as ESD followed by esophagectomy was.
Active surveillance and clinical response evaluation after chemoradiotherapy for esophageal cancer is now being assessed in the SANO trial (63). The aim is to avoid esophagectomy in patients with oncologic complete remission, as established in the Cross trial (64).
Our group was the first to report salvage ERs of small residual tumor after chemoradiotherapy in adenocarcinoma in the absence of lymph node metastases (65); This report demonstrates that curative treatment in patients unfit for surgery but fit enough for systemic sedated endoscopy is feasible. This research may contribute to a study on endoscopic surveillance programs with intention to select patients after chemoradiotherapy to be a candidate to perform salvage ER of remnant or recurrent carcinoma when unfit for surgical esophageal resection but fit enough for endoscopy.
Because of the effectiveness of RFA it will be difficult to improve on new ablation techniques. A candidate could by cryoablation. This treatment could be performed as primary ablation or in case of bad healing or non-responding after RFA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Inoue H, Endo M. Endoscopic esophageal mucosal resection using a transparent tube. Surg Endosc 1990;4:198-201. [Crossref] [PubMed]
- Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol 2008;103:788-97. [Crossref] [PubMed]
- Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut 2010;59:1169-77. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Yousef F, Cardwell C, Cantwell MM, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am J Epidemiol 2008;168:237-49. [Crossref] [PubMed]
- Desai TK, Krishnan K, Samala N, et al. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett's oesophagus: a meta-analysis. Gut 2012;61:970-6. [Crossref] [PubMed]
- Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017;49:191-8. [Crossref] [PubMed]
- Ishiguro S, Sasazuki S, Inoue M, et al. Effect of alcohol consumption, cigarette smoking and flushing response on esophageal cancer risk: a population-based cohort study (JPHC study). Cancer Lett 2009;275:240-6. [Crossref] [PubMed]
- Song BG, Min YW, Lee JH, et al. Efficacy and safety of endoscopic submucosal dissection in elderly patients with esophageal squamous cell carcinoma. Surg Endosc 2017;31:3905-11. [Crossref] [PubMed]
- Bugter O, van de Ven SEM, Hardillo JA, et al. Early detection of esophageal second primary tumors using Lugol chromoendoscopy in patients with head and neck cancer: A systematic review and meta-analysis. Head Neck 2019;41:1122-30. [Crossref] [PubMed]
- Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer 1998;83:220-31. [Crossref] [PubMed]
- Meyer V, Burtin P, Bour B, et al. Endoscopic detection of early esophageal cancer in a high-risk population: does Lugol staining improve videoendoscopy? Gastrointest Endosc 1997;45:480-4. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Bulsiewicz WJ, Dellon ES, Rogers AJ, et al. The impact of endoscopic ultrasound findings on clinical decision making in Barrett's esophagus with high-grade dysplasia or early esophageal adenocarcinoma. Dis Esophagus 2014;27:409-17. [Crossref] [PubMed]
- Fernandez-Sordo JO, Konda VJ, Chennat J, et al. Is Endoscopic Ultrasound (EUS) necessary in the pre-therapeutic assessment of Barrett's esophagus with early neoplasia? J Gastrointest Oncol 2012;3:314-21. [PubMed]
- Pech O, May A, Gunter E, et al. The impact of endoscopic ultrasound and computed tomography on the TNM staging of early cancer in Barrett's esophagus. Am J Gastroenterol 2006;101:2223-9. [Crossref] [PubMed]
- Pouw RE, Heldoorn N, Alvarez HL, et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc 2011;73:662-8. [Crossref] [PubMed]
- Wu HL, Guan BX, Liu B, et al. The intrapapillary capillary loop (IPCL) changes in superficial esophageal lesions. Dis Esophagus 2017;30:1-5. [PubMed]
- Inoue H, Kaga M, Ikeda H, et al. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol 2015;28:41-8. [PubMed]
- Li B, Chen H, Xiang J, et al. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2013;146:1198-203. [Crossref] [PubMed]
- van Vilsteren FG, Pouw RE, Alvarez HL, et al. Learning endoscopic resection in the esophagus. Endoscopy 2015;47:972-9. [Crossref] [PubMed]
- Peters FP, Kara MA, Rosmolen WD, et al. Stepwise radical endoscopic resection is effective for complete removal of Barrett's esophagus with early neoplasia: a prospective study. Am J Gastroenterol 2006;101:1449-57. [Crossref] [PubMed]
- Kato H, Haga S, Endo S, et al. Lifting of lesions during endoscopic mucosal resection (EMR) of early colorectal cancer: implications for the assessment of resectability. Endoscopy 2001;33:568-73. [Crossref] [PubMed]
- Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc 2011;74:35-43. [Crossref] [PubMed]
- Pouw RE, Beyna T, Belghazi K, et al. A prospective multicenter study using a new multiband mucosectomy device for endoscopic resection of early neoplasia in Barrett's esophagus. Gastrointest Endosc 2018;88:647-54. [Crossref] [PubMed]
- Scholvinck DW, Belghazi K, Pouw RE, et al. In vitro assessment of the performance of a new multiband mucosectomy device for endoscopic resection of early upper gastrointestinal neoplasia. Surg Endosc 2016;30:471-9. [Crossref] [PubMed]
- Alzoubaidi D, Graham D, Bassett P, et al. Comparison of two multiband mucosectomy devices for endoscopic resection of Barrett's esophagus-related neoplasia. Surg Endosc 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut 2017;66:783-93. [Crossref] [PubMed]
- Subramaniam S, Chedgy F, Longcroft-Wheaton G, et al. Complex early Barrett's neoplasia at 3 Western centers: European Barrett's Endoscopic Submucosal Dissection Trial (E-BEST). Gastrointest Endosc 2017;86:608-18. [Crossref] [PubMed]
- Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci 2014;59:1862-9. [Crossref] [PubMed]
- Wu Y, Zhang H, Zhou B, et al. Clinical efficacy of endoscopic submucosal dissection in the treatment of early esophageal cancer and precancerous lesions. J Cancer Res Ther 2018;14:52-6. [Crossref] [PubMed]
- Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010;72:255-64, 264.e1-2.
- Pech O, May A, Rabenstein T, et al. Endoscopic resection of early oesophageal cancer. Gut 2007;56:1625-34. [Crossref] [PubMed]
- Probst A, Aust D, Markl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015;47:113-21. [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-60. [Crossref] [PubMed]
- Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010;8:23-9. [Crossref] [PubMed]
- Phoa KN, Pouw RE, van Vilsteren FGI, et al. Remission of Barrett's esophagus with early neoplasia 5 years after radiofrequency ablation with endoscopic resection: a Netherlands cohort study. Gastroenterology 2013;145:96-104. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low-grade dysplasia in Barrett's esophagus and its implications for disease progression. Am J Gastroenterol 2000;95:3383-7. [Crossref] [PubMed]
- Curvers WL, Ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol 2010;105:1523-30. [Crossref] [PubMed]
- Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [Crossref] [PubMed]
- Phoa KN, Rosmolen WD, Weusten BLAM, et al. The cost-effectiveness of radiofrequency ablation for Barrett's esophagus with low-grade dysplasia: results from a randomized controlled trial (SURF trial). Gastrointest Endosc 2017;86:120-129.e2. [Crossref] [PubMed]
- Krishnadath KK, Wang KK, Taniguchi K, et al. Persistent genetic abnormalities in Barrett's esophagus after photodynamic therapy. Gastroenterology 2000;119:624-30. [Crossref] [PubMed]
- Hage M, Siersema PD. 5-aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barrett's oesophagus: a randomised trial. Gut 2004;53:785-90. [Crossref] [PubMed]
- Overwater A, Weusten BLAM. Cryoablation in the management of Barrett's esophagus. Curr Opin Gastroenterol 2017;33:261-9. [Crossref] [PubMed]
- van Munster SN, Overwater A, Haidry R, et al. Focal cryoballoon versus radiofrequency ablation of dysplastic Barrett's esophagus: impact on treatment response and postprocedural pain. Gastrointest Endosc 2018;88:795-803. [Crossref] [PubMed]
- Manner H, Neugebauer A, Scharpf M, et al. The tissue effect of argon-plasma coagulation with prior submucosal injection (Hybrid-APC) versus standard APC: A randomized ex-vivo study. United European Gastroenterol J 2014;2:383-90. [Crossref] [PubMed]
- Manner H, May A, Kouti I, et al. Efficacy and safety of Hybrid-APC for the ablation of Barrett's esophagus. Surg Endosc 2016;30:1364-70. [Crossref] [PubMed]
- Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett's epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6-12. [PubMed]
- Peerally MF, Bhandari P, Ragunath K, et al. Radiofrequency ablation compared with argon plasma coagulation after endoscopic resection of high-grade dysplasia or stage T1 adenocarcinoma in Barrett's esophagus: a randomized pilot study (BRIDE). Gastrointest Endosc 2018. [Epub ahead of print]. [PubMed]
- Bergman JJ, Zhang YM, He S, et al. Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus. Gastrointest Endosc 2011;74:1181-90. [Crossref] [PubMed]
- Haidry RJ, Butt MA, Dunn J, et al. Radiofrequency ablation for early oesophageal squamous neoplasia: outcomes form United Kingdom registry. World J Gastroenterol 2013;19:6011-9. [Crossref] [PubMed]
- Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch 2005;446:497-504. [Crossref] [PubMed]
- Scholvinck D, Kunzli H, Meijer S, et al. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc 2016;30:4102-13. [Crossref] [PubMed]
- Kunzli HT, Belghazi K, Pouw RE, et al. Endoscopic management and follow-up of patients with a submucosal esophageal adenocarcinoma. United European Gastroenterol J 2018;6:669-77. [Crossref] [PubMed]
- Manner H, Pech O, Heldmann Y, et al. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc 2015;29:1888-96. [Crossref] [PubMed]
- Kunzli HT, van Berge Henegouwen MI, Gisbertz SS, et al. Pilot-study on the feasibility of sentinel node navigation surgery in combination with thoracolaparoscopic lymphadenectomy without esophagectomy in early esophageal adenocarcinoma patients. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Motoori M, Yano M, Ishihara R, et al. Comparison between radical esophagectomy and definitive chemoradiotherapy in patients with clinical T1bN0M0 esophageal cancer. Ann Surg Oncol 2012;19:2135-41. [Crossref] [PubMed]
- Yamamoto S, Ishihara R, Motoori M, et al. Comparison between definitive chemoradiotherapy and esophagectomy in patients with clinical stage I esophageal squamous cell carcinoma. Am J Gastroenterol 2011;106:1048-54. [Crossref] [PubMed]
- Kawaguchi G, Sasamoto R, Abe E, et al. The effectiveness of endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer. Radiat Oncol 2015;10:31. [Crossref] [PubMed]
- Suzuki G, Yamazaki H, Aibe N, et al. Endoscopic submucosal dissection followed by chemoradiotherapy for superficial esophageal cancer: choice of new approach. Radiat Oncol 2018;13:246. [Crossref] [PubMed]
- Noordman BJ, Spaander MCW, Valkema R, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol 2018;19:965-74. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Noordzij IC, Curvers WL, Huysentruyt CJ, et al. Salvage endoscopic resection in patients with esophageal adenocarcinoma after chemoradiotherapy. Endosc Int Open 2018;6:E1126-9. [PubMed]


