
A faster detection method for high-sensitivity cardiac troponin—POCT quantum dot fluorescence immunoassay
Introduction
Acute myocardial infarction (AMI) is one of the major diseases that threaten human health. In recent years, the incidence of AMI has been increasing year by year (1-3). The early diagnosis of AMI is conducive to rapid diagnosis and treatment which can improve the prognosis of patients with AMI (4,5). High-sensitivity cardiac troponin (hs-cTn) can measure even low cTn concentration with high precision, which has important clinical significance for the diagnosis and risk stratification of AMI, and is a specific indicator for the diagnosis of AMI (2,6,7,8). According to the guidelines, if the results of hs-cTn did not increase in patients with chest pain at admission, blood sample should be taken again at intervals of 1 to 2 hours, and compared with the first results, if the results increased by more than 30%, the diagnosis of AMI should be considered (2,9). In addition, it has been shown that elevated troponin is associated with an increased risk of adverse outcome in patients with AMI (10). However, electrochemiluminescence is most commonly used to detect troponin at present. The method is currently detected on a large biochemical analyzer, and the time taken to detect hs-cTn is long, which means that it, s unable to perform bedside inspection and count against the rapid diagnosis of AMI (11-13). While the disadvantage of traditional Point of Care Testing (POCT) method is using serum, which is not possible at pre-hospital first aid. Therefore, the research of POCT quantum dot immunofluorescence based on whole blood is more valuable.
Quantum dot immunofluorescence immunoassay uses a double antibody sandwich method. The quantum dot-labeled antibody-antigen-coated antibody immune complex formed on the nitrocellulose membrane generates a fluorescent signal by exciting quantum dots, and the quantitative detection result is obtained by the instrument, which has the traits of high fluorescence efficiency, good stability, difficult to be quenched or gathered, and easy to surface modification, which is more conducive to pre-hospital first aid and early diagnosis (12,14). This study adopts POCT quantum dots immunofluorescence as a new method. Compared with the traditional Roche method, Vazyme POCT quantum dots immunofluorescence detection only needs to collect blood samples, and demands just 12 minutes to get results. This new method is characterized by simple operation, high-speed and is more propitious for rapid diagnosis of clinical emergency. Howbeit there is currently no research to evaluate the advantages of the new method. Therefore, we conducted a research to check the diagnostic performance of the new hs-troponin test performed in the whole blood sample and to compare with the Roche plasma sample detection in order to evaluate the advantages of this new detection method—POCT quantum dot immunofluorescence.
Methods
Study population
From August to November 2017, we prospectively recruited 415 consecutive patients in all who had chest pain from the outpatient and Emergency Department of the Affiliated Wuxi No. 2 People’s Hospital of Nanjing Medical University. A total of 5 mL sample of blood was drawn from patients using tubes containing heparin. This study was approved by the Ethics Committee of the Affiliated Wuxi No. 2 People’s Hospital of Nanjing Medical University and was carried out in compliance with the Helsinki Declaration. All subjects gave written informed consent.
Clinical evaluation
All patients underwent preliminary clinical evaluations, including the inquiries of medical history, physical examinations, device examinations and laboratory tests. In addition, the device examinations consisted of pulse oximetry, electrocardiogram, electrocardiogram monitoring, chest radiographs, echocardiography and ambulatory blood pressure monitoring and the laboratory tests included regular measurements of hs-troponin, creatine kinase and isozyme, myoglobin, brain natriuretic peptide precursor and creatinine from presentation to 24 hours after presentation. The patient’s diagnosis, treatment and evaluation items were determined by bedside doctors.
Research and analysis
After blood samples were collected from patients, heparin lithium anticoagulant whole blood was detected on the Vazyme AFS-1000 instrument, centrifugal plasma was detected on Roche’s Cobas 601.Cobas 601 was used as the reference system and Vazyme AFS 1000 was used as the pending evaluation system. The Roche machine was daily used calibration, quality controlled in control, ensured that no hemolysis, lipemia, jaundice, turbidity, record both results. When the diagnosis of AMI was established and the patient needed to be transferred to the catheter room or coronary care unit for treatment, continuous sampling was discontinued.
The upper reference value of Roche Cobas e601 analyzer and matching electrochemiluminescence hs-troponin T test kits, calibrators, quality control products was 14 ng/L (99th percentile) and the detection range was 3–10,000 ng/mL. The reference interval of Vazyme AFS-1000 Dry Fluorescence Detector and its matched Quantum Dot Fluorescent Immune High-sensitivity Cardiac Troponin I Test Strip (for serum, plasma, and whole blood)was ≤0.05 ng/mL, the range was 0.02–100 ng/mL. CK-MB and myoglobin were measured using an immunoassay (CK-MB, mass assay) (Elecsys 2010, Roche Diagnostics).
Judgement of the final diagnosis
Based on all available medical records, involving all clinical history, physical examination results, laboratory test results, and instrumental test results (consisting of hs-troponin but not the hs-troponin values used for this study), two cardiologists independently gave a diagnosis from these. Considering if there was a discrepancy in diagnoses, a third cardiologist would adjudicate and make the final decision.
Acute coronary syndrome (ACS) refers to the acute ischemic syndrome caused by unstable coronary atherosclerotic plaque rupture or erosion, which covers ST-segment elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infarction (NSTEMI) and unstable angina (UA), where STEMI and NSTEMI are collectively referred to as AMI. We were judged according to the latest global definition of myocardial infarction. The diagnosis of AMI and other predefined were determined by the 2017 ESC Guidelines for the Management of Acute Myocardial Infarction. The diagnostic criteria for AMI include a history of ischemic chest pain, dynamic changes in the electrocardiogram of myocardial ischemia and necrosis, and dynamic changes in serum myocardial biochemical markers (mainly hs-cTn, CK-MB), and the results of hs-cTn were based on the results of Roche electrochemiluminescence detection method (9-11) .
Statistical analysis
Continuous variables were presented as a mean ± standard deviation or median (interquartile range), and categorical variables were expressed as numbers and percentages. We constructed receiver operating characteristic (ROC) curves to analyze the diagnostic efficacy of Vazyme quantum dot immunofluorescence hs-cTn in AMI at different time points, selected the best diagnostic demarcation point and calculated the sensitivity of Vazyme hs-cTn in the diagnosis of AMI, specific Degree, positive likelihood ratio, negative likelihood ratio, positive predictive value, negative predictive value, and then compared area under the ROC curve (AUC) with the corresponding value of electrochemiluminescence hs-cTn. Data analysis was performed to use SPSS20.0 software (SPSS, Chicago, IL, USA) for statistical data processing. All hypothesis tests were two-tailed. P<0.05 was considered statistically significant.
Results
Characteristics of study subjects
The baseline characteristics of the study population can be shown in Table 1. The baseline values for all two hs-cTn tests in 415 consecutive patients were valid. Of the 415 suspected patients with the symptom of chest pain, 32 patients (7.7%) were eventually diagnosed with AMI, 26 patients (6.3%) with UA, 123 patients (29.6%) with coronary artery disease excepted myocardial infarction and UA. In addition, 103 patients (24.8%) were presented with the non-coronary disease, 104 patients (25.1%) were non-cardiogenic diseases, and the symptom of 27 patients (6.5%) were unexplained.
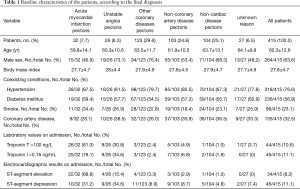
Full table
Evaluation of accuracy in diagnosing AMI
We compared the expression of three cardiac injury markers in the blood of patients with AMI-POCT quantum dot immunofluorescence hs-troponin I, electrochemiluminescence hs-troponin T and CK-MB for the sake of clarifying the accuracy of hs-troponin in the diagnosis of AMI. Therefore, the AUC and 95% CI of POCT quantum dot immunofluorescence hs-cTnI, electrochemiluminescence hs-cTnT and CK-MB in patients with AMI were calculated and the corresponding ROC curves were plotted. The graph is shown in Figure 1. The area under the curve (AUC) of POCT was 0.866, and the 95% CI was 0.783 to 0.949, it can be shown that the POCT quantum dot immunofluorescence hs-troponin assay had a high diagnostic efficiency in the diagnosis of AMI. The AUC of Roche electrochemiluminescence was 0.937, with a 95% CI of 0.898 to 0.976. There was no statistically significant difference between the two [Z value =1.527, P value =(0.063, 0.064)]. The AUC of CK-MB was 0.680. 95% CI ranged from 0.543 to 0.817. It can be seen that the diagnostic efficacy of the two hs-troponin test method was much higher than that of other myocardial injury markers. In addition, for 22 patients with ST-segment elevation AMI, we carried out an extra analyses that the AUC of POCT quantum dot immunofluorescence is 0.851 and electrochemiluminescence is 0.900 [Z value =0.039, P value = (0.348, 0.444)]. Both of them had a good diagnostic performance. The diagnostic efficiency of the two sensitivity analyses was not statistically different among gender, age, and other factors such as renal insufficiency.
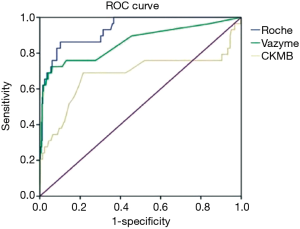
Performance evaluation of two methods
As an alternative diagnostic method, we define AMI based on hs-troponin levels. However, in patients with AMI, cTn began to release within 3 to 6 hours, a total of seven AMI patients was excluded within 3 hours. With a cut-off of electrochemiluminescence hs-cTnT >100 ng/L and POCT quantum dot immunofluorescence hs-CTnI >0.16 ng/mL as positive, we assessed the diagnostic of AMI and compared it with the final diagnosis. The final results can be seen in Figure 2. The clinical sensitivity of POCT quantum dot immunofluorescence was higher than that of electrochemiluminescence (88% vs. 84%). The specificity of POCT quantum dot immunofluorescence was slightly lower than that of electrochemiluminescence (93.7% vs. 93.9%). The positive predictive value of POCT quantum dot immunofluorescence was 47.8%, while the negative predictive value of it was 99.2%. The false positive rate of POCT quantum dot immunofluorescence was 6.20%, while the false negative rate of it was 12.0%. The positive predictive value of electrochemiluminescence was 47.7%, while the negative predictive value of it was 98.9%. The false positive rate of electrochemiluminescence was 6.0% and the false negative rate of it was 16.0%. Our results indicated that POCT quantum dot immunofluorescence hs-troponin test method had almost the same diagnostic efficiency as electrochemiluminescence. On the other hand, it can also be demonstrated in patients with AMI, Vazyme POCT quantum dot immunofluorescence hs-troponin detection can replace Roche electrochemiluminescence as an independent biomarker of myocardial injury for the diagnosis of AMI.
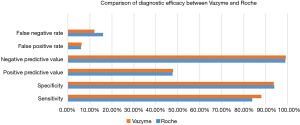
Diagnostic efficacy in cardiogenic diseases
Firstly, we measured the ROC curve for cardiogenic patients. ROC curve can be seen in Figure 3 (POCT quantum dot immunofluorescence AUC 0.607, 95% CI: 0.551 to 0.664; electrochemiluminescence AUC 0.634, 95% CI: 0.578 to 0.690). For the diagnosis of cardiogenic diseases, the negative predictive value of the negative test results of the POCT quantum dot immunofluorescence and electrochemiluminescence hs-troponin assays (defined as below the 99th percentile, less than 10% imprecision) were 34.2% and 33.7%, respectively. Thus it can be seen that the accuracy of hs-troponin detection in differentiating cardiogenic and non-cardiac diseases is only moderate, but the difference between the two hs-troponin test methods was not significant. Secondly, on the basis of cardiogenic diseases, we analyzed the differences between POCT quantum dot immunofluorescence and electrochemiluminescence hs-troponin in UA and coronary related diseases. We found that for patients with UA, the negative predictive values were separately 95.1% and 95.2%, which have high diagnostic efficiency. Moreover, electrochemiluminescence was significantly better in predicting patients with coronary artery disease [area under the operating curve (AUC) =0.677], compared with POCT quantum dot immunofluorescence (AUC =0.619). Although the diagnostic accuracy was not high, there was still no significant difference [Z value =1.626, P value = (0.063, 0.064)].
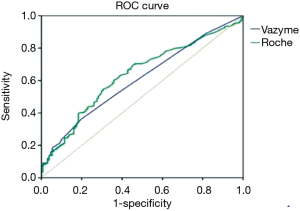
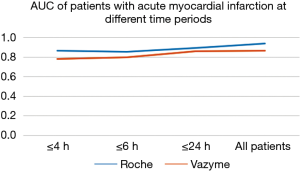
Diagnostic efficacy of AMI patients at different time periods
Through analyzing the hs-troponin levels in patients with AMI at different time points, we calculated the ROC curve and AUC for POCT quantum dot immunofluorescence and electrochemiluminescence, separately. For example, in the time period less than or equal to 4 h, the AUC of POCT quantum dot immunofluorescence was 0.783 and electrochemiluminescence was 0.863, as the specific data can be seen in Figure 4. From this, we could analyze hs-troponin levels at different time periods, there was little difference observed between the two test methods, and the diagnostic efficacies were both high.
Discussion
In our study, we separately analyzed and compared the diagnostic efficacy and difference of 415 patients with POCT quantum dot immunofluorescence and electrochemiluminescence hs-troponin detection methods on admission, in order to make an early diagnosis of AMI and to further evaluate the advantages of the new POCT quantum dot immunofluorescence technique. First of all, POCT quantum dot immunofluorescence and electrochemiluminescence hs-troponin detection methods were excellently correlated, besides the AUCs of patients with AMI are 0.866 and 0.937, and they all had an excellent diagnostic performance. Although the diagnostic efficiency of POCT quantum dot immunofluorescence was slightly lower than that of electrochemiluminescence, the electrochemiluminescence test took a long time to get a result and was not propitious to early diagnosis of AMI. By contrast, the POCT quantum dot immunofluorescence test method has a number of advantages such as quick and easy, portable instrument and so on. An interesting finding of the current study is that by simply collecting whole blood samples, a similar result to electrochemiluminescence plasma samples can be achieved. What is more, according to the analysis of patients with AMI at different time periods, it can be seen from Figures 2-4 that diagnostic efficiency of POCT quantum dot immunofluorescence was stable and the diagnostic performance was better than that of other markers of myocardial injury such as CK-MB. Combined with ECG and other tests, POCT quantum dot immunofluorescence hs-troponin detection can be used as a separate myocardial infarction marker for diagnostic analysis, and will greatly improve the detection rate of AMI. Thirdly, POCT quantum dot immunofluorescence hs-troponin detection is more effective in emergency patients. For patients with chest pain, the rapid and efficient detection method is particularly important. POCT quantum dot immunofluorescence hs-troponin assay can merely use whole blood to analyze results within twelve minutes. The matching instrument is small in size and easy to detect. It is more suitable for emergency patients with chest pain. Fourth, in the patients admitted to the hospital with suspected AMI, POCT quantum dot immunofluorescence hs-troponin detection assay can reliably exclude most non-AMI patients on the basis of initial measurements. The specificity of POCT quantum dot immunofluorescence hs-troponin detection was 93.7% and the negative predictive value was 99.2%, which was a bit higher than that of electrochemiluminescence. When the POCT quantum dot immunofluorescence hs-troponin method combined with clinical performance and electrocardiogram, the proportion of patients with an unclear diagnosis of chest pain after the first troponin test will be greatly reduced, which will greatly save the cost of examination (15) .
Despite the risk of UA is far lower than that of AMI and the prognosis is much better than that of AMI, UA is an early manifestation of AMI and belongs to the same category as an ACS. Although treatment has progressed at the moment, but the risk of cardiac events in patients still exists. The most appropriate treatment is to control the occurrence of cardiac events in the short term, and it mainly depends on the identification and distinction of risk (16). In addition, along with the increase of serum hs-cTn levels, the incidence of cardiac events with UA also increases, showing that the hs-troponin level is of great significance for the prognosis of patients with UA. Nonetheless, POCT quantum dot immunofluorescence and electrochemiluminescence hs-troponin detections are not very accurate for other cardiac diseases, the current value of hs-cTn is limited to the differential diagnosis of AMI. Further study is meaningful for the identification of biomarkers that reliably detect myocardial ischemia rather than AMI.
In addition, when the hs-troponin level is elevated but there is no clinical manifestation of ischemia, demanding to be combined with electrocardiogram and other tests, we should look for other reasons that can cause elevated levels of hs-troponin, such as myocarditis, respiratory failure, acute heart failure, kidney failure and so on (16,17). Though the sensitivity of electrocardiogram is on the low side and the diagnostic accuracy of hs-troponin in the differential diagnosis of myocardial injury is much higher than the electrocardiogram, the electrocardiogram can immediately identify the ST-segment elevation AMI (18).
The current study carries inherent limitations. In the first place, we conducted a prospective study that failed to quantify the clinical effect of the diagnosis. Therefore, it is necessary to carry out intervention studies to obtain additional information such as final prognosis and follow-up examination. Secondly, in the wake of the increasing sensitivity of hs-troponin assays, the probability of false positives is greatly increased. This will augment panic among doctors and patients and will disturb the doctor’s judgment. How to increase the sensitivity of hs-troponin detection and reduce false positive is the focal point of our follow-up research. Thirdly, some patients who are positive for POCT quantum dot immunofluorescence and electrochemiluminescence hs-troponin detections are ultimately classified as having non-AMI. These patients may exist a small myocardial infarction or be interfered with the diagnosis of AMI by other diseases such as pulmonary embolism. This result may lead to the underestimation of the specificity of the POCT quantum dot immunofluorescence hs-troponin assay.
In conclusions, our findings highlight the diagnostic challenge of ACS and suggest that POCT quantum dot immunofluorescence assay for hs-troponin detection is more simple and rapid than electrochemical immunoassay, which is more suitable for rapid diagnosis. Therefore, the POCT quantum dot immunofluorescence hs-troponin assay can be used as an independent diagnosis of AMI.
Acknowledgements
Funding: This work was supported by grant from Natural Science Foundation of Jiangsu Province (No. BK20171147), The Science and Technology Projects of Wuxi City (WX18IVJN016), The Major Science and Technology Projects of Wuxi City (Z201804).
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of the Affiliated Wuxi No. 2 People’s Hospital of Nanjing Medical University and was carried out in compliance with the Helsinki Declaration. All subjects gave written informed consent.
References
- Shi QX, Cai KX. Neurology, Clinical Diagnosis and Treatment of Acute Myocardial Infarction Complicated with Cerebral Infarction in Elderly Patients. World Latest Medicine Information 2016;16:10-1.
- Carlton E, Greenslade J, Cullen L, et al. Evaluation of High-Sensitivity Cardiac Troponin I Levels in Patients With Suspected Acute Coronary Syndrome. JAMA Cardiol 2016;1:405-12. [Crossref] [PubMed]
- Tukish OV, Okrugin SA, Yunusova EY, et al. Acute myocardial infarction in elderly and senile patients: epidemiology study according to the who program "registry of acute myocardial infarction. Adv Gerontol 2016;29:123-7. [PubMed]
- Casals G, Filella X, Augé JM, et al. Impact of ultrasensitive cardiac troponin I dynamic changes in the new universal definition of myocardial infarction. Am J Clin Pathol 2008;130:964-8. [Crossref] [PubMed]
- Gaze DC. High-sensitive cardiac troponin assays: application for prime-time use. Biomark Med 2010;4:341-3. [Crossref] [PubMed]
- Omland T. New sensitive cardiac troponin assays for the early diagnosis of myocardial infarction. Drugs Today (Barc) 2011;47:303-12. [Crossref] [PubMed]
- Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 2010;31:2197-204. [Crossref] [PubMed]
- McCann CJ, Glover BM, Menown IB, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J 2008;29:2843-50. [Crossref] [PubMed]
- SEC Working Group for the 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting with ST-segment Elevation:, Alfonso F, Sionis A, et al. Comments on the 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting With ST-segment Elevation. Rev Esp Cardiol (Engl Ed) 2017;70:1039-45.
- Jia GC, Zhang AM. High-sensitive troponin I test in clinical value for early diagnosis of acute myocardial infarction. Jilin Medical Journal 2014;35:4837-8.
- Lauridsen KG, Revsholm J, Løfgren B. Appropriate Use of High-Sensitivity Cardiac Troponin Levels in Patients With Suspected Acute Myocardial Infarction. JAMA Cardiol 2017;2:228-9. [Crossref] [PubMed]
- Yuan R, Cheng F, Zhang XB. Application of Quantum-Dot-Based Immunofluorescence for the Special Detection of Bladder Cancer Cell Line BIU-87. Medical Journal of Wuhan University 2017;38:28-30, 35.
- Omland T, de Lemos JA, Sabatine MS, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 2009;361:2538-47. [Crossref] [PubMed]
- Peacock WF 4th, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117-26. [Crossref] [PubMed]
- Yang LY, Rong HU, Huang Y. Clinical Significance of Myoglobin Combined with Troponin in Early Diagnosis of Acute Myocardial Infarction. Chinese & Foreign Medical Research 2016;14:63-4.
- Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin Chem 2009;55:2098-112. [Crossref] [PubMed]
- Eggers KM, Jaffe AS, Lind L, et al. Value of cardiac troponin I cutoff concentrations below the 99th percentile for clinical decision-making. Clin Chem 2009;55:85-92. [Crossref] [PubMed]
- Oyama MA, Sisson DD. Cardiac troponin-I concentration in dogs with cardiac disease. J Vet Intern Med 2004;18:831-9. [Crossref] [PubMed]


