
Exploring the molecular and biological background of lung neuroendocrine tumours
Lung neuroendocrine tumours (LNET) include an extremely heterogeneous group of malignancies, whose spectrum of either morphological or clinical characteristics varies from well-differentiated and indolent to poorly differentiated and fatal diseases. In the last decades, the increase in LNET incidence was probably related to an improvement in diagnostic tools (especially histology and immunohistochemistry) and lung cancer awareness, as well as to the wider diffusion of screening programs. LNET represent approximately 20% of all the neuroendocrine neoplasms of the human body and include four histological variants namely typical carcinoid (TC), atypical carcinoid (AC), large-cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC) (1). SCLC is the most common subtype, representing 20% of invasive primary lung cancers; LCNEC accounts for approximately 3% of resected lung tumours; bronchial carcinoids (TC and AC) are about 2% to 0.2% of all the primary lung cancers (2). Current WHO classification criteria require accurate mitotic count and the assessment of the presence of necrosis to define the four main types of lung neuroendocrine neoplasms (1). This four-tier histological spectrum of LNET fits clinically into a three-tier prognostic scheme. Moreover, some histological (spindle cell, oncocytic and melanocytic) and morphological variants are included in each main LNET histotype (1), complicating differential diagnosis and therapeutic choices. The diagnosis of a suspected LNET typically begins by confirming the neuroendocrine differentiation, using a panel of general immunohistochemical (IHC) markers including chromogranin A, CD56 and synaptophysin. Nevertheless, these tumour markers are not able to distinguish among different subtypes of LNET (3), while the Ki67 antigen may play a significant role. In this regard, to date the only validate diagnostic meaning of Ki67 is related to the differentiation of TC/AC from SCLC, but a lung-specific grading system based on evaluation of this marker has been proposed (4).
From a clinical point of view, carcinoids (TC and AC) are differentiated from carcinomas (LCNEC and SCLC). TC are low-grade tumours and affected patients usually have a long life expectancy, AC are intermediate-grade tumours with varying clinical behaviours, while LCNEC and SCLC are generally grouped together as high-grade malignancies with a similar dismal prognosis (1,5,6). Nevertheless, as for the histological features, exceptions exist also in terms of clinical behavior. Indeed, the prognosis associated with LCNEC is less defined. This subtype is considered a highly aggressive disease, but a wide range of 5-year survival rate (15–57%) is reported (7) with a weighted mean of 34%. The wide range of reported clinical behavior of LCNEC probably reflects the heterogeneity in both inclusion criteria and treatment approaches emerging from various studies.
The treatment approaches to LNET subtypes are markedly different, which underscores the importance of an accurate pathologic diagnosis. Current recommendations according to ENETS guidelines [2015] (8) include surgery as first choice in localized carcinoids, while for locally advance/metastatic disease a multidisciplinary approach integrating the available treatment options (as somatostatin analogues, chemotherapy, everolimus, peptide receptor radionuclide therapy and surgery) is recommended (9). Due to their low incidence and histological/biological heterogeneity, no unique treatment strategy exists for LCNEC and these patients are usually treated with either non-small cell lung cancer (NSCLC) or SCLC chemotherapy protocols (10). Regarding SCLC, it usually represents a nonsurgical disease, featured by a high response rate to platinum-etoposide chemotherapy (with or without radiotherapy), counteracted by early relapse and disappointing results to second-line treatments (11). The recent introduction of immunotherapy represents a promising perspective in extended-stage SCLC, although the identification of reliable predictive biomarkers still represents an unmet need (12).
With the final aim to correlate molecular/expression profile of surgically-resected LNET with their prognosis and therefore attempt to establish a more effective treatment strategy, Ichiki et al. performed an IHC analysis of neuroendocrine markers and programmed cell death-ligand 1 (PD-L1) in 105 pulmonary NET. The PD-L1 expression did not significantly predict prognosis, while a trend correlating the higher expression of the neuroendocrine markers chromogranin A and synaptophysin with a worse survival outcome was reported, leading the authors to speculate that these IHC analyses may assist clinicians in the decision about adjuvant chemotherapy or follow-up intervals after surgery (13). Although the limited and retrospective nature, this study provided new insights about LNET with potential clinico-therapeutical implications to be further validated.
With a similar intent, a series of studies applied innovative molecular biology techniques in these rare and heterogeneous diseases in order to improve diagnosis and development of new therapeutics (Table 1). In 2012, Peifer et al. published the first integrative analysis on SCLC (14), reporting in all cases inactivation of TP53 and RB1 and recurrent mutations in CREBBP, EP300 and MLL genes that encode histone modifiers. This study implicated histone modification as a major feature of SCLC, revealing potentially therapeutically tractable genomic alterations. In 2015, George et al. reported a deeper comprehensive genomic profiling of SCLC (15), confirming concurrent inactivation of TP53 and RB1 and highlighting a tumour suppressive role for NOTCH gene family. Analysis also identified frequent losses in chromosome 3p arm, homozygous deletion of CDKN2A gene and amplification involving MYC gene family. They further discovered recurrent expression of p73Δex2/3 suggesting its possible role as oncogene in this LNET subtype. The Clinical Lung Cancer Genome Project was the first that reported a direct comparison among all lung cancer subtypes (16). In this study, carcinoids, SCLC and NSCLC were analysed using comprehensive genomic and transcriptomic analysis and the results highlighted specific alterations for each subtype. In detail, carcinoid tumours (considered as homogeneous without distinction between typical and atypical subtypes) represent a class of stable genomic neoplasms where mutations and copy number variations (CNV) are rare events. On the other side, SCLC showed a genomic profile more similar to NSCLC in terms of frequent mutations in TP53 gene, but a peculiar inactivation of RB1 and CNV affecting FHIT, MYCL1 and MYCN genes. Of note, in this study emerged for the first time as LCNEC represent a heterogeneous and distinct group of large cell lung tumours. Deep investigation using transcriptomic analysis suggested that LCNEC were very similar to SCLC in both featuring genomic alterations and clinical behaviour. Subsequently, Fernandez-Cuesta et al. performed a direct and deep analysis focused on pulmonary carcinoids (17). Results showed recurrent and exclusive mutations in genes involved in chromatin remodelling as MEN1, PSIP1 and ARID1A. Moreover, comparative data using expression profile in carcinoids, lung adenocarcinomas and SCLC suggested that carcinoids are a completely different group of tumours not or only in part related to the other two subtypes. Therefore, the authors concluded indicating as their findings support a model where pulmonary carcinoids do not represent early progenitor lesions of other neuroendocrine tumours, such as SCLC or LCNEC, but arise through independent cellular mechanisms. In contrast to this assertion, in 2016 Simbolo et al. performed genomic analysis on a cohort of LNET including all the four histotypes defined by WHO classification (18). Results showed a strong linkage between the four histological subtypes in terms of mutations affecting chromatin-remodelling genes, which were similarly altered in all cases. In addition, an increment in inactivating events affecting TP53 and RB1 genes together with a decrement in number of mutations affected MEN1 gene was observed going from the low-grade subtype (TC) to the high-grade (LCNEC and SCLC), suggesting an evolutionary model of tumour progression for these neuroendocrine neoplasms. In the same study, new molecular and prognostic markers were identified such as MEN1 in AC, KMT2D in SCLC and RB1 for all subtypes. In the same year, Rekhtman et al. published an interesting study focused on the heterogeneous group of LCNEC (19). Three different molecular subgroups were described: SCLC-like, NSCLC-like and carcinoid-like. In detail, while the SCLC-like cases showed co-alteration of TP53 and RB1 genes, the NSCLC-like group harboured mutations in TP53 gene with concurrent mutations in KRAS and/or STK11 genes. Finally, the carcinoid-like group (only two cases) had mutations only in MEN1 gene. George and colleagues obtained similar results in their recent study published in 2018 (20). A genomic and transcriptomic analysis were performed on 75 LCNEC identifying three molecular subtypes. In particular, results showed a strong linkage to SCLC, similarly characterized by concurrent mutations in TP53 and RB1 genes, high expression of ASCL1 and DLL3 and low expression of NOTCH gene family. This group was so called SCLC-like. The other LCNEC were divided in “type I” and “type II” groups. The first was characterized by mutations in TP53, STK11 and/or KEAP1 with high expression of ASCL1 and DLL3 and low expression of NOTCH gene family. The second group included LCNEC characterized by concurrent mutations in TP53 and RB1 with low expression of ASCL1 and DLL3 and high expression of NOTCH gene family.
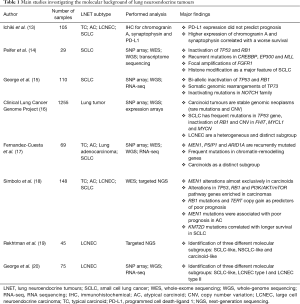
Full table
In conclusions, technological and scientific achievements obtained in the last 10 years improved our knowledge about rare neoplasms, as LNET. These discoveries are contributing to better define optimal diagnostic and therapeutical strategies, sometimes highlighting the limitations of past dogmas that should change after the upcoming evidence.
Acknowledgements
Funding: E Bria and S Pilotto were supported by the Italian Association for Cancer Research—My First AIRC Grant 2013 (No. 14282) and are supported by AIRC-IG 20583.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th edition. Lyon: IARC, 2015.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628-38. [PubMed]
- Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol 2010;21 Suppl 7:vii65-71. [Crossref] [PubMed]
- Pelosi G, Rindi G, Travis WD, et al. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol 2014;9:273-84. [Crossref] [PubMed]
- Asamura H, Kameya T, Matsuno Y, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. J Clin Oncol 2006;24:70-6. [Crossref] [PubMed]
- Beasley MB, Thunnissen FB, Brambilla E, et al. Pulmonary atypical carcinoid: predictors of survival in 106 cases. Hum Pathol 2000;31:1255-65. [Crossref] [PubMed]
- Iyoda A, Makino T, Koezuka S, et al. Treatment options for patients with large cell neuroendocrine carcinoma of the lung. Gen Thorac Cardiovasc Surg 2014;62:351-6. [Crossref] [PubMed]
- Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604-20. [Crossref] [PubMed]
- Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine Tumors of the Lung: Current Challenges and Advances in the Diagnosis and Management of Well-Differentiated Disease. J Thorac Oncol 2017;12:425-36. [Crossref] [PubMed]
- Hiroshima K, Mino-Kenudson M. Update on large cell neuroendocrine carcinoma. Transl Lung Cancer Res 2017;6:530-9. [Crossref] [PubMed]
- Rossi A, Tay R, Chiramel J, et al. Current and future therapeutic approaches for the treatment of small cell lung cancer. Expert Rev Anticancer Ther 2018;18:473-86. [Crossref] [PubMed]
- Pacheco J, Bunn PA. Advancements in Small-cell Lung Cancer: The Changing Landscape Following IMpower-133. Clin Lung Cancer 2019;20:148-60.e2. [Crossref] [PubMed]
- Ichiki Y, Matsumiya H, Mori M, et al. Predictive factors of postoperative survival among patients with pulmonary neuroendocrine tumor. J Thorac Dis 2018;10:6912-20. [Crossref] [PubMed]
- Peifer M, Fernandez-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- A genomics-based classification of human lung tumors. Sci Transl Med 2013;5:209ra153. [PubMed]
- Fernandez-Cuesta L, Peifer M, Lu X, et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun 2014;5:3518. [Crossref] [PubMed]
- Simbolo M, Mafficini A, Sikora KO, et al. Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D. J Pathol 2017;241:488-500. [Crossref] [PubMed]
- Rekhtman N, Pietanza MC, Hellmann M, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res 2016;22:3618-29. [Crossref] [PubMed]
- George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun 2018;9:1048. [Crossref] [PubMed]


