Paravertebral catheter analgesia for minimally invasive Ivor Lewis oesophagectomy
Introduction
Oesophagectomies are performed by an open or a minimally invasive fashion. They are associated with severe morbidity which can be contributed by severe post-operative pain (1,2). Severe pain, which is one of the most important factors after oesophagectomy, may in the early post-operative phase result in changes in respiratory function and pulmonary mechanisms. Poor pain management will result in inability to cough or expectorate, or even take a deep breath. This will result in retained secretions, atelectasis, and culminating in chest infection (3). Therefore, pain contributes to the development of respiratory complications. Adequate pain relief will improve postoperative pulmonary functions and reduce pulmonary complications (4,5). In addition, pain management has an important role in relieving anxiety, enhancing early mobilization and recovery, and thereby reducing postoperative complications and hospital stay (6-8). For decades epidural catheter analgesia has been the gold standard for oesophagectomy. Recently, paravertebral catheter analgesia (PVCA) was introduced as an appropriate alternative for patients undergoing an oesophagectomy.
Epidural analgesia
Thoracic epidural has been the gold standard approach for optimal analgesia in patients undergoing an open oesophagectomy, and even minimally invasive oesophagectomy (MIO). Epidural has proven advantages over systemic analgesia after thoraco-abdominal oesophagectomy (9). However, epidural has several disadvantages too. Firstly, epidural has risk for technical failure due to misplacement, secondary migration of a catheter after correct placement, occlusion, or suboptimal dosing (10). Secondly, epidural has significant risks for developing epidural hematoma, epidural abscess and neurological damage (11). Thirdly, not all patients can have an epidural catheter due to contra-indications such as an impaired coagulation and previous spinal operation. Fourthly, epidural analgesia also impair postoperative recovery due to both urinary retention and impaired mobility, which negatively influence postoperative recovery or enhanced recovery after surgery (ERAS) programmes (12). Finally, concerns have been raised that epidural induces post-operative hypotension, resulting in the need for more intravenous fluid and vasoconstrictors, such as noradrenaline. The vasoconstrictors may result in impaired submucosal microcirculatory blood flow and the perfusion of the gastric conduit (13). Diminished blood flow in the gastric conduit may impact on the integrity of the anastomosis or result in gastric conduit necrosis.
Postoperative pain and recovery are also influenced by the surgical approach for oesophagectomy. MIO is increasingly performed worldwide and recent studies showed that even a hybrid MIO approach (laparoscopic approach followed by right thoracotomy), as well as a robot-assisted MIO, resulted in a significantly reduction of post-operative pain as well as pulmonary complications when compared to open esophagectomy (14-16). As a consequence, optimization of the postoperative analgesia is essential. A recent report which studied the effect of epidural in patients who underwent a robot-assisted oesophagectomy showed that epidural analgesia was insufficient in almost half of the patients (17). In 44% of the patients the epidural analgesia was terminated before the planned 5 day postoperative. Early termination was caused by complication or negligible effect. In addition, this report stated a complete sensory block in 49% and 30% of the patients at day 1 and 4 postoperative, respectively.
PVCA
The use of PVCA is a promising alternative to epidural analgesia in patients undergoing an open or minimally invasive oesophagectomy. PVCA can be achieved easily by catheter placement under direct vision or percutaneously guided with ultrasound. It is more difficult to insert it percutaneously guided with ultrasound, or even blindly without ultrasound, especially in the obese patients or those with osteophytes around the spine.
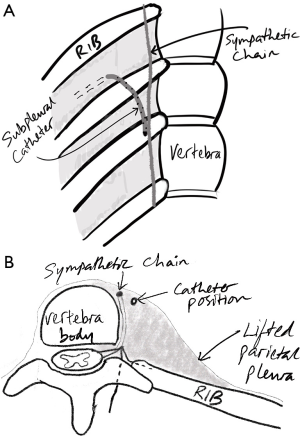
The para-vertebral space is a wedge-shaped space, which lies on either side of the vertebral column and contains intercostal nerves (ICNs) and the sympathetic chain. Placement of analgesia within this paravertebral space results in unilateral somatic and sympathetic nerve blockade (18). The paravertebral spread of local anaesthesia results in continuous extra-pleural ICN block. PVCA can be applied in by different techniques, as described above. During thoracic surgery, a percutaneous paravertebral thoracic catheter is easily inserted under direct vision. This has been first described by Sabanathan et al. (19). Video-assisted placement during video-assisted thoracoscopy has also been described first in 1994 (20). We place our PVCA under direct vision at the end of the TMIO. The catheter was inserted successfully in all cases, without a haematoma or infection post operatively.
Epidural analgesia versus paravertebral catheter analgesia
Systematic reviews suggest that the analgesia provided by a thoracic epidural or a paravertebral have equal outcomes in patients who had a thoracotomy (5). There was no significant difference between PVCA and epidural analgesia at 4–8, 24, and 48 hours {weighted mean difference (WMD) 0.37 [95% confidence interval (CI), (−0.5 to 121), 0.05 (−0.6 to 0.7), −0.04 (−0.4 to 0.3)]} respectively. Some clinical trials, which compared PVCA with a thoracic epidural for post-thoracotomy pain relief, showed worse pain relief in patients with PVCA (21). However, other studies comparing open thoracotomy with video-assisted thoracoscopic surgery showed that there is an equal analgesic effect after PVCA, when compared with a thoracic epidural catheter (22-25). A propensity-scored matched analysis showed no difference regarding length of stay or complications in 648 patients who underwent a thoracotomy with thoracic epidural analgesia or PVCA (26). A multicentre randomized controlled trial (RCT) in Netherlands comparing epidural catheter analgesia versus a PVCA is starting, and it may answer the question as to which postoperative analgesia is favourable in patients who had TMIO or RAMIE. The important endpoint in this RCT is a validated patient reported outcome questionnaire (QoR-40) that looks at the quality of recovery in a patient.
Currently, there is no level one evidence about the optimal pain management after open and minimally invasive oesophagectomy. A recent meta-analysis analysing pain management after oesophagectomy showed no difference in postoperative pain scores between systemic and epidural analgesia at 24 hours [mean difference (MD) 0.89; 95% CI, −0.47 to 2.24) and 48 hours (MD 0.15; 95% CI, −0.60 to 0.91)] (27). In addition, no significant differences were found regarding pulmonary complications between systemic and paravertebral analgesia [relative risk (RR) 1.49; 95% CI, 0.31 to 7.12]. Most of the patients in the included studies in this meta-analysis underwent an open oesophagectomy. In the biggest series of MIO ever reported from UPMC, Pittsburgh by Luketich, the patients only received intraoperative ICN blocks and PCA postoperatively, without any epidural or PVCA (28). We present a prospective analysis of PVCA for 100 consecutive patients who underwent a totally minimal invasive Ivor Lewis oesophagectomy for oesophageal cancer, with intraoperative ICN analgesia, PCA and spinal analgesia in an ERAS setting.
Our experience with PVCA for minimally invasive Ivor Lewis oesophagectomy.
Methods
The oesophago-gastric cancer unit of the Norfolk and Norwich University Hospital is a high volume center performing specialized upper gastrointestinal tract surgery. One hundred consecutive patients undergoing a totally minimal invasive oesophagectomy (TMIO) between 2012 and 2017 were analysed from a single institution. Patients followed an established standardized peri-operative ERAS pathway.
All patients underwent staging and preoperative assessment guided by a specialist multidisciplinary cancer team. The staging involved upper gastrointestinal endoscopy with biopsy, computerized tomographic scan and positron emission tomographic scan of the chest, abdomen, and pelvis. In addition, patients with Siewert 2 or 3 cancers underwent a staging laparoscopy before neoadjuvant treatment. The fitness of a patient for surgery was assessed by walking the patient up two floors, and using cardiopulmonary exercise testing. Patients who could walk up 2 floors under 1 minute without stopping to rest were deemed fit. Patients received perioperative chemotherapy or neoadjuvant chemoradiotherapy if the tumour was stages as T2 or more, or any locoregional nodes were involved (29,30).
Totally intravenous anaesthesia was used with remifentanil in combination with propofol were used for the general anaesthesia. Target controlled infusion according to the BIS monitor (abbreviated EEG) from NAP5 project, using Marsh model for propofol and Minto model for remifentanil, was used. Intravenous paracetamol (1 gm) and magnesium (5 gm) were also given. During the thoracic phase single lung ventilation was achieved with a double lumen endotracheal tube. The TMIO consists of a laparoscopic gastric mobilization, formation of a narrow gastric conduit, and locoregional lymphadenectomy, followed by a right-sided thoracoscopic oesophageal mobilization and intra-thoracic lymphadenectomy in the left lateral decubitus position. A high intra-thoracic (above azygous venous arch) end-to-side oesophago-gastric anastomoses was performed in with a circular stapler (CDH 29, Ethicon Endosurgery) through a 5 cm mini-thoracotomy (the access port was also used to remove the specimen). All patients received standard perioperative antibiotic and thromboembolic prophylaxis. A nasogastric tube to decompress the gastric conduit was inserted at the end. A basal (28French) right sided chest drain and a Jackson-Pratt bulb suction drain in the base of the left chest (inserted from the right chest), were used.
Patients received a right-sided paravertebral catheter in the right chest, in combination with right sided ICN analgesia and a patient-controlled analgesia (PCA), at the end of the TMIO. A spinal analgesia with morphine was placed before induction of the anaesthesia, or when the patient was turned in the left lateral decubitus position after the abdominal phase of TMIO. Before any skin incision in the chest phase for the 5 cm access port was made, two levels of ICN block were placed by the surgeon with a total of 20 mL of 0.25% levobupivacaine (Chirocaine) with a 21-gauge green needle. This was at the intercostal space at the presumed 5 cm access port level, and at the intercostal space immediately above that of the access port. At the end of the TMIO procedure, and before closing the access port wound, the paravertebral catheter was inserted under vision at the intercostal space one level above the access port wound (20). A 16-gauge Tuohy needle (Epidural Minipack System 1, Smiths Medical, Ashford, UK) was inserted into the intercostal space pointing caudally and medially, just above the lower rib and 3 cm lateral to the spinous process of the vertebral column. Under vision, the needle was advanced into the subpleural space with the bevel tip of the needle pointing caudally. The needle tip is carefully pushed medially towards the paravertebral area, in the subpleural space, and being careful to avoid puncturing the pleura. Then, 20 mL of saline was quickly injected from a 20 mL syringe into the subpleural space to lift the parietal pleura in the paravertebral region, and to create a big subpleural space. Subsequently, the catheter was inserted via the Tuohy needle and tunnelled under the pleura under direct vision into the paravertebral space. The tip of the catheter should sit next to the sympathetic chain (Figure 1). The system was completed with the catheter filter and connector, and then loaded with 30 to 40 mL of 0.25% levobupivacaine. The catheter was secured to the skin with a Lockit Plus epidural catheter securement device (Smiths Medical). In the theatre recovery, 0.125% levobupivacaine was started through the paravertebral catheter with an infusion rate of 15–20 mL/hour. In addition, patients were provided with a PCA with 1 mg morphine bolus with 5-minute lockout and a maximum dose of 10 mg. The paravertebral catheter was routinely removed at the 4th or 5th day postoperatively.
Patients were extubated at the end of the case and transferred to high dependency care for one night. From high-dependency unit (HDU), the discharge to the specialized oesophago-gastric ward was dependent on hemodynamic and respiratory stability. During the postoperative period, patients routinely received intravenous paracetamol four times a day till day 3. At day 3, paracetamol was changed to oral suspension 1 gm four times a day.
Peri-operatively, the patient followed a standardized ERAS program. This integrated care pathway program covers all aspects regarding pain management, mobilization, feeding, and fluid management. The patient starts respiratory exercises (deep breathing exercises and three coughs in succession) immediately postoperative in the recovery area, and starts both mobilization and respiratory exercises under the supervision of a physiotherapist from day 1 postoperatively, till they are independent. Patients start feeding at day 1 postoperative when they will be allowed to start 30 mL an hour of high calorie liquid nutrition (Fresubin Jucy, Fresenius Kabi, Runcorn, UK). This will be gradually built up till a soft diet over the following days.
Pain was monitored and scored using a verbal descriptive scale on a scale of 0 to 3 (Table 1). Data were all prospectively and independently recorded using a standardized pain score document. Pain scores were recorded by specialist pain nurses, as well as by trained nursing staff, every four to six hours. Physical observations were also recorded every hourly, while PCA were utilised. Patients were independently reviewed and their pain scored 4–6 times a day. If pain was poorly controlled (>2), the pain scores and observations were obtained more frequently. Pain scores were documented in the patient record. Complications regarding the pain management were assessed and recorded daily.

Full table
If pain scores were >2, rescue medication was administered. Rescue medication at the bedside consisted of an ICN block at 2 levels with 20 mL of 0.5% Chirocaine. This was injected in the intercostal space of and the intercostal space immediately above the 5 cm access wound site.
Statistical analysis
Data were collected on Microsoft Excel (2010) and analysed with IBM SPSS statistics (v.22.0, Armonk, NY, USA). Descriptive statistics are presented.
Results
Basic demographics
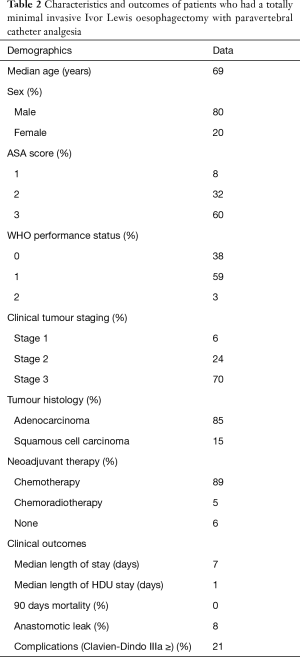
The median age of the patient was 69 years, and 94% of the patients were stage 2 or 3 (Table 2). Neoadjuvant perioperative chemotherapy or chemoradiation therapy was administered in 94% of the patients. Most patients were ASA score 2 or 3 (92%). Their WHO performance status was 0 or 1 in 38 and 59% cases, respectively. The clinical stages of the tumours were 24% stage 2 and 70% stage 3.
Full table
Analgesia use
In 94% of cases, administration of spinal morphine was feasible. But in 6% of cases, it was difficult to administer due to patient condition such as recent stoppage of anticoagulation or previous spinal surgery.
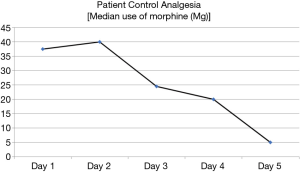
The use of PCA was highest in the first 2 days postoperatively and the frequency of PCA used gradually reduced over the subsequent 3 days (Figure 2).
A paravertebral catheter was inserted in 100% cases. There were only 4 paravertebral catheter failures postoperatively—two catheters were disconnected before day 4 and were removed, one fell out on day 3, and one was removed on day 3 because it was leaking.
Pain score
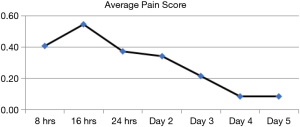
The median pain score across each of the 5 days was 0. The average pain score when the patients were scored at 8 hourly interval over the first 24 hours post operatively were 0.44, 0.55 and 0.37 (Figure 3). The average pain score on day 2, 3, 4 and 5 post operatively were 0.34, 0.21, and 0.09 and 0.08, respectively.
Other postoperative outcomes
Eleven patients (11%) received rescue medication postoperative. Seven patients required right sided ICN blocks with 20 mLs 0.5% Chirocaine on the ward to relieve pain from the right sided mini-thoracotomy access wound site—four patients required it on day 2, and the other three patients on day 3 post operatively. Four patients had one dose 50 mg Voltarol per rectum on day 2 and 3 post operatively. Nineteen patients (19%) received intravenous Metaraminol or Noradrenaline, as vasoconstrictors, on the first day postoperative. In addition, 3% and 0% received Metaraminol or Noradrenaline at day 2 and 3 postoperative, respectively. The doses of vasoconstrictors received were very low (2–5 mLs/hour single dose) in all cases, and they were given in the HDU setting. As part of our ERAS protocol, the urine catheter was removed on day 2 or 3 postoperatively. Only in 3 patients a re-catheterisation was necessary y due to bladder retention. Pneumonia occurred in 21% of patients post operatively.
Discussion
In this single center cohort study, PVCA is part of the standard protocol in patients undergoing a TMIO. We used the PVCA routinely because of its ease of insertion, effective analgesic properties, and minimal side effects when compared with an epidural. In addition, it does not restrict ERAS programmes, it improves patient mobility, and the urine catheter can be safely removed on day 2 or 3 without significant urine retention. In our cohort, only 3 patients needed re-catheterisation. Early removal of the urine catheter enhances the early mobilisation of the patient.
In our experience of 100 consecutive patients having TMIO, there were only 4 minor complications with the use of PVCA, all due to catheter related failure. None of these resulted in any severe morbidity to the patient, and the rescue medications gave adequate analgesia subsequently. Rescue medication was used only in 11% of the cases. The use of an epidural in patients who underwent a robot-assisted oesophagectomy resulted in rescue medication (intravenous opioids) in 45% of the patients in the first 4 days postoperative (17), and the median time for removal of urine catheter was day 6 postoperatively.
One of the disadvantages for epidural analgesia is postoperative hypotension, which can reduce the perfusion of the gastric conduit (13). Epidural analgesia use in robot-assisted minimally invasive oesophagectomy is associated with 48% of the patients receiving intravenous noradrenaline on day 1 postoperatively. We demonstrate that only 19% of the patients who underwent a TMIO received noradrenaline at day 1 postoperative. As hypotension has potential side effects after oesophagectomy this is an important beneficial effect of PVCA compared with epidural analgesia. Hypotension in patients with epidural are often treated with intravenous fluid resulting in fluid overload and oedematous tissues, or it is treated with intravenous vasoconstrictor which impacts on the perfusion of the gastric conduit.
Another potential pain management strategy is injection of liposomal bupivacaine formulation (Exparel) into the paravertebral or subpleural space. Exparel (Pacira Pharmaceuticals) is a liposome with an extended release of bupivacaine and therefore requires only one per-operative administration by the surgeon to achieve post-operative pain relief. In addition, no catheter is required and hence the patient will not be bound to a drip pole, which can impede the patient’s mobility. The liposomal formulation of bupivacaine has a duration of action of 72 hours (31). A retrospective study observed a significantly improved pain management in the early postoperative days after a single shot of Exparel peri-operatively compared with epidural analgesia in patients who underwent a thoracotomy (32) However, Exparel ($300 per vial) is significantly more expensive than bupivacaine HCL ($3 per vial).
The possible long-term effect of the para-vertebral analgesia, which could be achieved by ICN protection or decreased nociceptive input, has also been studied. Currently there is an ongoing RCT comparing the effectiveness of a PVCA versus a thoracic epidural in reducing chronic post-thoracotomy pain (33).
Conclusions
We conclude that PVCA is highly effective for the management of pain after a TMIO. Therefore, pain management with PVCA is a good alternative to epidural catheter analgesia for minimally invasive oesophagectomy.
Acknowledgements
This was supported by Dr. K Al-Naimi, Dr. N Saunders, and Dr. J Francis, consultant anaesthetists from the Department of Anaesthesia at the Norfolk and Norwich University Hospital.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was performed at the Norfolk and Norwich University Hospital, and all investigators ensure that the principles of the Economic and Social Research Council have been followed. Briefly, these principles ensure quality and integrity of research, respect confidentiality and anonymity, require informed consent, avoid harm, ensure independent and impartial research, and ensure that participants participate voluntarily.
References
- Gottschalk A, Cohen SP, Yang S, et al. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594-600. [Crossref] [PubMed]
- Furrer M, Rechsteiner R, Eigenmann V, et al. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg 1997;12:82-7. [Crossref] [PubMed]
- Sabanathan S, Eng J, Mearns AJ. Alterations in respiratory mechanics following thoracotomy. J R Coll Surg Edinb 1990;35:144-50. [PubMed]
- Richardson J, Sabanathan S, Jones J, et al. A prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth 1999;83:387-92. [Crossref] [PubMed]
- Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth 2006;96:418-26. [Crossref] [PubMed]
- Weijs TJ, Ruurda JP, Nieuwenhuijzen GA, et al. Strategies to reduce pulmonary complications after esophagectomy. World J Gastroenterol 2013;19:6509-14. [Crossref] [PubMed]
- Richardson J, Sabanathan S, Mearns AJ, et al. Efficacy of pre-emptive analgesia and continuous extrapleural intercostal nerve block on post-thoracotomy pain and pulmonary mechanics. J Cardiovasc Surg (Torino) 1994;35:219-28. [PubMed]
- Cense HA, Lagarde SM, de Jong K, et al. Association of no epidural analgesia with postoperative morbidity and mortality after transthoracic esophageal cancer resection. J Am Coll Surg 2006;202:395-400. [Crossref] [PubMed]
- Flisberg P, Tornebrandt K, Walther B, et al. Pain relief after esophagectomy: Thoracic epidural analgesia is better than parenteral opioids. J Cardiothorac Vasc Anesth 2001;15:282-7. [Crossref] [PubMed]
- Hermanides J, Hollmann MW, Stevens MF, et al. Failed epidural: causes and management. Br J Anaesth 2012;109:144-54. [Crossref] [PubMed]
- Christie IW, McCabe S. Major complications of epidural analgesia after surgery: results of a six-year survey. Anaesthesia 2007;62:335-41. [Crossref] [PubMed]
- Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology 1995;82:1474-506. [Crossref] [PubMed]
- Al-Rawi OY, Pennefather SH, Page RD, et al. The effect of thoracic epidural bupivacaine and an intravenous adrenaline infusion on gastric tube blood flow during esophagectomy. Anesth Analg 2008;106:884-7. table of contents. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- Kingma BF, Visser E, Marsman M, et al. Epidural analgesia after minimally invasive esophagectomy: efficacy and complication profile. Dis Esophagus 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001;95:771-80. [Crossref] [PubMed]
- Sabanathan S, Smith PJ, Pradhan GN, et al. Continuous intercostal nerve block for pain relief after thoracotomy. Ann Thorac Surg 1988;46:425-6. [Crossref] [PubMed]
- Soni AK, Conacher ID, Waller DA, et al. Video-assisted thoracoscopic placement of paravertebral catheters: a technique for postoperative analgesia for bilateral thoracoscopic surgery. Br J Anaesth 1994;72:462-4. [Crossref] [PubMed]
- Tamura T, Mori S, Mori A, et al. A randomized controlled trial comparing paravertebral block via the surgical field with thoracic epidural block using ropivacaine for post-thoracotomy pain relief. J Anesth 2017;31:263-70. [Crossref] [PubMed]
- Kosinski S, Fryzlewicz E, Wilkojc M, et al. Comparison of continuous epidural block and continuous paravertebral block in postoperative analgaesia after video-assisted thoracoscopic surgery lobectomy: a randomised, non-inferiority trial. Anaesthesiol Intensive Ther 2016;48:280-7. [PubMed]
- Okajima H, Tanaka O, Ushio M, et al. Ultrasound-guided continuous thoracic paravertebral block provides comparable analgesia and fewer episodes of hypotension than continuous epidural block after lung surgery. J Anesth 2015;29:373-8. [Crossref] [PubMed]
- Kobayashi R, Mori S, Wakai K, et al. Paravertebral block via the surgical field versus epidural block for patients undergoing thoracotomy: a randomized clinical trial. Surg Today 2013;43:963-9. [Crossref] [PubMed]
- Bimston DN, McGee JP, Liptay MJ, et al. Continuous paravertebral extrapleural infusion for post-thoracotomy pain management. Surgery 1999;126:650-6; discussion 6-7. [Crossref] [PubMed]
- Blackshaw WJ, Bhawnani A, Pennefather SH, et al. Propensity score-matched outcomes after thoracic epidural or paravertebral analgesia for thoracotomy. Anaesthesia 2018;73:444-9. [Crossref] [PubMed]
- Visser E, Marsman M, van Rossum PS, et al. Postoperative pain management after esophagectomy: a systematic review and meta-analysis. Dis Esophagus 2017;30:1-11. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Bergese SD, Ramamoorthy S, Patou G, et al. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res 2012;5:107-16. [Crossref] [PubMed]
- Khalil KG, Boutrous ML, Irani AD, et al. Operative Intercostal Nerve Blocks With Long-Acting Bupivacaine Liposome for Pain Control After Thoracotomy. Ann Thorac Surg 2015;100:2013-8. [Crossref] [PubMed]
- Yeung J, Melody T, Kerr A, et al. Randomised controlled pilot study to investigate the effectiveness of thoracic epidural and paravertebral blockade in reducing chronic post-thoracotomy pain: TOPIC feasibility study protocol. BMJ Open 2016;6:e012735. [Crossref] [PubMed]