
Perioperative outcome of lung cancer surgery in women: results from a Spanish nationwide prospective cohort study
Introduction
The epidemiology of lung cancer is changing in many areas or the world in terms of incidence differences by gender (1). Tobacco smoking is the major cause, accounting for 80% of the worldwide lung cancer burden in males and at least 50% in females (2). However, in the last three decades, lung cancer incidence rates have decreased or leveled off among men, but have risen among women (1,3-5). This increase in women (by 600% in the last 50 years) has been defined as a ‘contemporary epidemic’ (6). Other factors rather than smoking and linked to sex may increase women’s susceptibility to lung cancer, including genetic predisposition, exposure to sex hormones, tumor histology and molecular profile (7,8). In addition, several lifestyle and behavioral factors related to smoking habits or environmental and occupational exposures could account for some sex-specific differences (9).
Lung cancer in women has been an increased focus of research, in particular prognosis and treatment outcomes as compared to men. It has been shown that women exhibit greater survival rates regardless of stage, histology, treatment modality or smoking status, even after adjusting for gender-specific life expectancy (10,11). Also, non-smoking status predicts better survival and possibly better response to therapy (12). The etiology behind these observations is theorized to be related to genetic and molecular differences (13) as well as to decreased DNA repair mechanisms, which is thought to lead to increased response to systemic chemotherapy (10). Moreover, better survival in older women may be related to higher prevalence of comorbidities among men of the same age (14).
Despite extensive evidence outlining gender differences in lung cancer, studies with special focus on differences by sex regarding perioperative outcomes are scarce. In an analysis of the Society of Thoracic Surgeon’s General Thoracic Database for all patients undergoing resection for lung cancer between 2002 and 2010, in which a total of 34,188 patients (16,643 men and 17,545 women) were considered, significant differences in postoperative complications and in-hospital and 30-day mortality favoring women were found (15). Other studies have demonstrated that male sex is a predictor of prolonged hospital stay and postoperative pulmonary and cardiac complications following surgery (16,17).
To add further evidence of potential differences in the perioperative outcome between men and women undergoing lung cancer surgery, a nationwide prospective cohort study was conducted. The objective of the study was to assess differential characteristics and postoperative complications of lung cancer surgery in women as compared to men, as well as to determine possible causal factors.
Methods
Study design
This was a retrospective analysis of data collected prospectively in the framework of a national registry set up by the Group of Postoperative Complications of the Spanish Society of Thoracic Surgeons (GCP-SECT). For the period of time between June 1, 2012 and November 30, 2014, data of 3,307 patients treated with some type of pulmonary resection due to bronchogenic carcinoma were systematically and prospective collected in the GCP-SECT database. A total of 24 thoracic surgery departments each belonging to a different hospital throughout Spain participated in the study. The study was approved by the Ethics Committee of each participating hospitals. Patients provided written informed consent prior to study participation.
Patients
Patients with a histological diagnosis of bronchogenic carcinoma who had undergone surgery with curative intent at the Thoracic Surgery Departments of the participating hospitals in the GCP-SECT project were included in the study. Surgical procedures included wedge lung resection, segmentectomy, lobectomy, bilobectomy or pneumonectomy. Patients undergoing exploratory video-assisted thoracoscopies or thoracotomies, life-saving emergency surgeries and cases with synchronous tumors were excluded. The indication of extent of resection was decided by the primary surgeon.
Data collection
For each patient, data collected were grouped into (I) preoperative general characteristics; (II) tumor-related variables; (III) surgery data; and (IV) postoperative data. Preoperative general characteristics of patients included age, sex, ECOG performance status (18), medical history [smoking, chronic obstructive pulmonary disease (COPD), tuberculosis, obstructive sleep apnea (OSA), atelectasis, pneumonitis, ischemic heart disease, atrial fibrillation, valve heart disease, hypertension, peripheral vascular disease, diabetes mellitus, obesity (body mass index, BMI >25 kg/m2)], stroke, and results of lung function tests. Tumor-related variables included histological type and TNM stage (2009 TNM Classification, 7th edition). Surgery-related data were type of approach, side, type of resection, and intraoperative complications (hypoxia, hemorrhage, arrhythmia, and rib fracture). Postoperative data were length of hospital stay and postoperative complications. Postoperative complications included atelectasis, pneumonitis, hemoptysis, reintubation, pleural effusion, air leak, arrhythmias, pulmonary edema, myocardial infarction, hemorrhage, bronchopleural fistula, pleural empyema, surgical wound infection, and surgical wound dehiscence. Severity of complications was graded according to Clavien-Dindo classification (19). Ninety-day mortality was also recorded.
These data were compiled consecutively and anonymously in a computerized database with online internet access, and the information was recorded in an Excel file stored by the GCP-SECT. All the participating hospitals have followed the guidelines of the Patient Personal Data Protection Law.
Statistical analysis
For the purpose of the present analysis, patients were stratified according to sex in order to examine differential characteristics between men and women. Categorical data are expressed as frequencies and percentages and quantitative data as mean and standard deviation (SD) or median with 95% confidence interval (CI). The Fisher’s exact test was used for the comparison of categorical variables between men and women, and the Student’s t-test for the comparison of continuous variables. In order to determine independent variables associated with lung cancer in women, variables that were found to be statistically significant in the bivariate analysis were included in a stepwise logistic regression model, in which gender (female vs. male) was the dependent variable. Statistical significance was set at P<0.05. Analyses were carried out using the Statistical Package for the Social Sciences (SPSS) version 9.4 for Windows.
Results
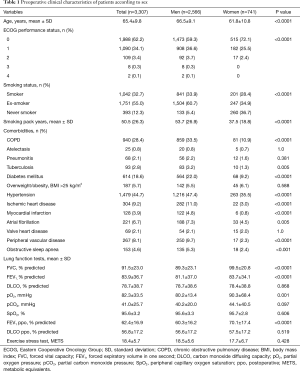
Of the 3,307 patients included in the GCP-SECT database during the study period, 2,566 (77.6%) were men and 741 (22.4%) women, with a mean (SD) age of 65.4 (9.8) years. Men were significantly older than women [66.5 (9.1) vs. 61.8 (10.8) years, P<0.0001]. The distribution of general preoperative characteristics in men and women is shown in Table 1. Women as compared with men showed a better ECOG performance status (e.g., grade 0, 72.1% vs. 59.3%); a lower percentage of current smokers (28.4% vs. 33.9%) and ex-smokers (34.9% vs. 60.7%) as well a higher percentage of never smokers (36.7% vs. 5.4%); a fewer number of associated comorbidities, including COPD, diabetes, overweight/obesity, hypertension, ischemic heart disease, myocardial infarction, peripheral vascular disease, and OSA; and higher forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and carbon monoxide diffusing capacity (DLCO). All these differences were statistically significant.
Full table
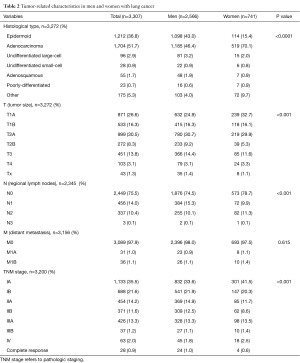
TNM categories, pathologic tumor stages, and histological types are shown in Table 2. There were statistically significant differences between men and women in histological type of tumor (with fewer percentages of epidermoid carcinoma and higher percentages of adenocarcinoma in women), tumor size (higher percentage of T1A in women: 32.7% vs. 24.9% in men), nodal status (higher percentage of N0 in women: 78.7% vs. 74.5% in men), and TNM staging (higher percentage of stage IA in women: 41.5% vs. 33.6% in men).
Full table
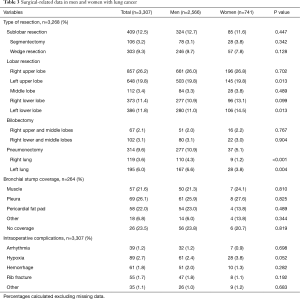
In relation to surgical-related data, differences in surgical approach and the side of surgery were not found. Video-assisted thoracoscopy (VATS) procedure was used in 30.4% of men and in 34.3% of women, and thoracotomy in 68.5% of men and 65.1% in women. Details of type of lung resection and intraoperative complications are shown in Table 3. A significantly higher percentage of men underwent pneumonectomy as compared with women (10.9% vs. 5.1%, P<0.001). Differences in coverage of the bronchial stump, extended resections or intraoperative complications were not found.
Full table
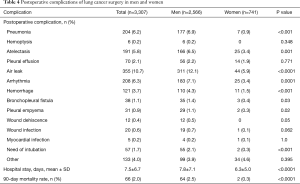
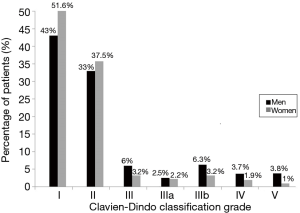
Overall, postoperative complications were more frequent in men than in women, with statistically significant differences in the rates of pneumonitis, atelectasis, air leak, arrhythmia, hemorrhage, bronchopleural fistula, pleural empyema, wound dehiscence, and need of intubation (Table 4). Significant differences in the severity of postoperative complications classified according to the Clavien-Dindo score were also observed, with higher percentages of women having grade I, and lower percentages having grades III, IIIa, IIIb, IV, and V as compared with men (P<0.001) (Figure 1).
Full table
The mean length of hospital stay was 7.8 (7.1) days in men and 6.3 (5.0) days in women (P<0.0001). This longer length of stay was mainly related to air leaks. The 90-day mortality rate was significantly lower in women as compared with men (0.3% vs. 2.5%, P<0.0001). A total of 109 patients (8.6% men vs. 2.8% women, P<0.001) were readmitted to the hospital within 30 days of primary surgery, with a mean length of stay of 9.2 (7.8) days for men and 5.6 (4.1) for women (P=0.158).
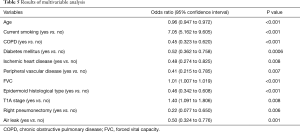
Results of the logistic regression analysis are shown in Table 5. It was found that women were younger than men, were more likely to be non-smokers, had better FVC values, more stage T1A tumors, and lower incidence of COPD, diabetes mellitus, ischemic heart disease, and peripheral vascular disease as well as epidermoid tumors, right pneumonectomy, and air leaks. Other variables were not significant in the multivariable analysis.
Full table
Discussion
This multicenter nationwide prospective cohort study of a large sample of patients with lung cancer undergoing surgical treatment with curative intent shows that the perioperative profile is better in women than in men. Notably, women were younger and had a lower rate of concomitant chronic diseases as compared with men. Also there were more women non-smokers than men. Differences in histology, with adenocarcinoma as the most common tumor in women were also remarkable. Postoperative complications and 30-day mortality were lower in women too.
The more favorable perioperative profile in women might be related to the consistent evidence of superior response to lung cancer treatment and survival benefit in women reported in numerous studies, including systematic reviews and meta-analysis (4,11,20-25). Also, longer survival duration and response to chemotherapy in patients with untreated early-stage lung cancer have been reported (26), suggesting that lung cancer has a different natural history among women. This survival advantage appears to be multifactorial, with hormonal influences, genetic, metabolic, lifestyle and environmental factors (27,28) other than smoking paying a role. However, although cancer mortality predictions for 2015 in the EU showed an overall favorable trend, with an estimated 26% fall in men and 31% in women since 1988, a 9% rise in lung cancer rates were expected, becoming the cancer with the highest rate, reaching and possibly overtaking breast cancer was predicted (29). In a comprehensive global analysis of lung mortality in women using the World Health Organization’s Cancer Mortality Database covering 65 populations on six continents, rates among young women (30–49 years) were stable or declining in 47 of 52 populations examined, whereas in older women (50–74 years) rates were increasing for more than half of populations examined, including most countries in Southern, Eastern, and Western Europe and South America (30). In this line, changes in aging of the population the risk factors for cancer could contribute 37.9% to the burden of lung cancer mortality expected to be observed among Spanish women during 2018–2022 (31). Interestingly, according to Bayesian prediction of lung cancer mortality among women in Spain, by 2020 lung cancer mortality rates may exceed those of breast cancer for ages 55–74 years, possibly because of the prevalence of smoking among women, and the screening for and more effective treatment of breast cancer (32).
In our study, women presenting with lung cancer were significantly younger than men even though the rate of active smokers was lower and they had smoked fewer cigarettes. Women appear to have an increased susceptibility cancer-causing chemicals or carcinogens in tobacco than men (33,34). Nicotine appears to trigger the gastrin releasing peptide receptor gene, which is also more active in women (35).
Chronic concomitant diseases were less frequently present in women than in men, but comorbidity does not appear to have an independent prognostic effect in patients with non-small cell lung cancer (36). Other findings of our study, such as a better performance status on diagnosis, higher percentage of women with IA stage (N0 78.7% in women vs. 74.5% in men, P<0.001) and adenocarcinoma as the most common histological type are consistent with previous studies (5,9,15). It is unclear why women are more predisposed to adenocarcinoma than men. Given that small-cell cancer cases are related to heavier smoking history, low exposure to cigarettes but increased sensitivity to their carcinogens may explain why the incidence of adenocarcinoma is higher among women (20). Also, estrogen may affect molecular and histological features of lung cancer, and it may explain some of the differences between genders (4,6,7).
The higher rate of pneumonectomies in men as compared with women found in the present study is consistent with data of other clinical series (37,38). A high rate of pneumonectomies worsens postoperative outcome with an increased risk of complications and negative impact on the quality of life. Indeed, in our study, women were less likely to develop postoperative complications as compared with men. The most frequent complication was air leak, which was observed in 12.1% of men and 5.9% of women. Postsurgical atelectasis occurred in 6.5% of men and 3.4% of women. It has been reported that these complications are associated with patient age, being more frequent over 70 years of age than in yours age groups (39). This lower rate of postoperative complications among women was associated with a shorter duration of hospitalization. The mortality rate at 30 days was also significantly lower in women.
Conclusions
The results of this multicenter nationwide study of lung cancer surgery with curative intent show that the perioperative profile is better in women than in men. Further studies focused on the epidemiology and biology of lung cancer may lead to improvement of patient-specific management according to gender in the future.
Acknowledgements
The authors thank all members of the Group of Postoperative Complications of the Spanish Society of Thoracic Surgeons (GCP-SECT) for their contribution to the study and Marta Pulido, MD, PhD, for editing the manuscript and editorial assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the participating centers involved in the Postoperative Complications Study Group of the Spanish Society of Thoracic Surgery (GCP-SECT). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016;893:1-19. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- De Matteis S, Consonni D, Pesatori AC, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol 2013;177:601-12. [Crossref] [PubMed]
- Remon J, Molina-Montes E, Majem M, et al. Lung cancer in women: an overview with special focus on Spanish women Clin Transl Oncol 2014;16:517-28. [Crossref] [PubMed]
- Janssen-Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer 2003;41:245-58. [Crossref] [PubMed]
- Patel JD, Bach PB, Kris MG. Lung cancer in US women: a contemporary epidemic. JAMA 2004;291:1763-8. [Crossref] [PubMed]
- Barrera-Rodriguez R, Morales-Fuentes J. Lung cancer in women. Lung Cancer (Auckl) 2012;3:79-89. [Crossref] [PubMed]
- Bae JM, Kim EH. Hormonal replacement therapy and the risk of lung cancer in women: an adaptive meta-analysis of cohort studies. J Prev Med Public Health 2015;48:280-6. [Crossref] [PubMed]
- Pauk N, Kubík A, Zatloukal P, et al. Lung cancer in women. Lung Cancer 2005;48:1-9. [Crossref] [PubMed]
- North CM, Christiani DC. Women and lung cancer: what’s new? Semin Thorac Cardiovasc Surg 2013;25:87-94. [Crossref] [PubMed]
- Nakamura H, Ando K, Shinmyo T, et al. Female gender is an independent prognostic factor in non-small-cell lung cancer: a meta-analysis. Ann Thorac Cardiovasc Surg 2011;17:469-80. [Crossref] [PubMed]
- Fu JB, Kau TY, Severson RK, et al. Lung cancer in women: analysis of the national Surveillance, Epidemiology, and End Results database. Chest 2005;127:768-77. [Crossref] [PubMed]
- Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 2005;128:370-81. [Crossref] [PubMed]
- Stabile LP, Siegfried JM. Sex and gender differences in lung cancer. J Gend Specif Med 2003;6:37-48. [PubMed]
- Tong BC, Kosinski AS, Burfeind WR, et al. Sex differences in early outcomes after lung cancer resection: Analysis of the Society of Thoracic Surgeons’ General Thoracic Database. J Thorac Cardiovasc Surg 2014;148:13-8. [Crossref] [PubMed]
- Wright CD, Gaissert HA, Grab JD, et al. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk-adjustment model. Ann Thorac Surg 2008;85:1857-65. [Crossref] [PubMed]
- Rueth NM, Parsons HM, Habermann EB, et al. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg 2012;143:1314-23. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Ulas A, Tokluoglu S, Kos M, et al. Lung cancer in women, a different disease: survival differences by sex in Turkey. Asian Pac J Cancer Prev 2015;16:815-22. [Crossref] [PubMed]
- Isla D, Majem M, Viñolas N, et al. A consensus statement on gender perspective in lung cancer. Clin Transl Oncol 2017;19:527-35. [Crossref] [PubMed]
- Visbal AL, Williams BA, Nichols FC 3rd, et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4,618 patients diagnosed between 1997 and 2002. Ann Thorac Surg 2004;78:209-15. [Crossref] [PubMed]
- Belani CP, Marts S, Schiller J, Socinski MA. Women and lung cancer: epidemiology, tumor biology, and emerging trends in clinical research. Lung Cancer 2007;55:15-23. [Crossref] [PubMed]
- Nakamura K, Ukawa S, Okada E, et al. Characteristics and prognosis of Japanese male and female lung cancer patients: The BioBank Japan Project. J Epidemiol 2017;27:S49-57. [Crossref] [PubMed]
- Sakurai H, Asamura H, Goya T, et al. Survival differences by gender for resected non-small cell lung cancer: a retrospective analysis of 12,509 cases in a Japanese Lung Cancer Registry study. J Thorac Oncol 2010;5:1594-601. [Crossref] [PubMed]
- Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol 2007;25:1705-12. [Crossref] [PubMed]
- Kubik A, Zatloukal P, Tomasek L, et al. A case-control study of lifestyle and lung cancer associations by histological types. Neoplasma 2008;55:192-9. [PubMed]
- Kubík AK, Zatloukal P, Tomásek L, et al. Dietary habits and lung cancer risk among non-smoking women. Eur J Cancer Prev 2004;13:471-80. [Crossref] [PubMed]
- Malvezzi M, Bertuccio P, Rosso T, et al. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol 2015;26:779-86. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. International variation in lung cancer mortality rates and trends among women. Cancer Epidemiol Biomarkers Prev 2014;23:1025-36. [Crossref] [PubMed]
- Clèries R, Buxó M, Martínez JM, et al. Contribution of changes in demography and in the risk factors to the predicted pattern of cancer mortality among Spanish women by 2022. Cancer Epidemiol 2016;40:113-8. [Crossref] [PubMed]
- Martín-Sánchez JC, Clèries R, Lidón C, et al. Bayesian prediction of lung and breast cancer mortality among women in Spain (2014-2020). Cancer Epidemiol 2016;43:22-9. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators. Henschke CI, Yip R, Miettinen OS. Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 2006;296:180-4. [Crossref] [PubMed]
- Haugen A. Women who smoke: are women more susceptible to tobacco-induced lung cancer? Carcinogenesis 2002;23:227-9. [Crossref] [PubMed]
- Shriver SP, Bourdeau HA, Gubish CT, et al. Sex-specific expression of gastrin-releasing peptide receptor: relationship to smoking history and risk of lung cancer. J Natl Cancer Inst 2000;92:24-33. [Crossref] [PubMed]
- Janssen-Heijnen MLG, Smulders S, Lemmens VE, et al. Effect of comorbidity on the treatment and prognosis of elderly patients with non-small cell lung cancer. Thorax 2004;59:602-7. [Crossref] [PubMed]
- de Perrot M, Licker M, Bouchardy C, et al. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg 2000;119:21-6. [Crossref] [PubMed]
- Foeglé J, Hédelin G, Lebitasy MP, et al. Specific features of non-small cell lung cancer in women: a retrospective study of 1738 cases diagnosed in Bas-Rhin between 1982 and 1997. J Thorac Oncol 2007;2:466-74. [Crossref] [PubMed]
- Cañizares Carretero MÁ, García Fontán EM, Blanco Ramos M, et al. Is age a predisposing factor of postoperative complications after lung resection for primary pulmonary neopasms? Cir Esp 2017;95:160-6. [PubMed]


