
Minimal invasive extracorporeal circulation (MiECC): the state-of-the-art in perfusion
Introduction
From the dawn of open-heart surgery in the early 1950s, a sequence of significant scientific achievements rendered cardiac surgery as a routine practice in the treatment of heart diseases. Contemporary advances in cardiac surgery including surgical techniques, anaesthesia and intensive care management markedly improved clinical outcomes. However, cardiac surgery is still hampered by considerable morbidity and subsequent mortality, especially in complex and high-risk procedures. As evidenced in the largest cardiac surgery registry (STS database) that incorporates data from over 200,000 procedures, operative results are excellent irrespective of surgical technique in the setting of low-risk elective coronary artery bypass grafting (CABG), isolated aortic valve replacement (AVR) or mitral valve repair (1). However, significant operative mortality and major morbidity (approaching the rate of 30%) is still observed not only in high-risk cases such as emergency CABG or acute aortic dissection repair, but also in the most common scenario of an isolated mitral valve replacement (MVR) or an elective combined procedure like AVR+CABG and MVR+CABG (Table 1). This literally means that in real-world one out of three patients may experience a serious postoperative complication in this setting.

Full table
The quest for optimal perfusion
When considering morbidity related to cardiac surgery not as an inevitable side effect but as a serious ongoing problem, then focus should be given on further advancing intraoperative management not just regarding surgery but for all other participating disciplines in the operating room (OR), that is anaesthesia and perfusion. However, it has to be primarily appreciated that optimal intraoperative perfusion, utilizing advanced cardiopulmonary bypass (CPB) technology, stands for the base of a favourable postoperative result. This parameter though is usually underestimated or even deliberately neglected by most cardiac surgeons. This attitude, in turn, leads to the point that the two of the three main stakeholders in the cardiac OR (the medical ones, that is surgery and anesthesia) continuously advance their performance level and hence their results, while perfusion (the non-medical discipline) remains just technical and thereby non-scientific in most circumstances. Thus, there is immense need to implement in cardiac OR all contemporary advancements in perfusion technology and monitoring so as to best apply cardiovascular physiology in clinical practice. This would ultimately upgrade the perfusionists from technicians to clinical perfusionists.
The role of MiECC
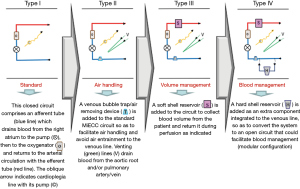
To underline the need for optimal intraoperative perfusion in cardiac surgery, one of the most eminent textbooks in perfusion (on bypass: advanced perfusion techniques, by Mongero and Beck) highlighted a decade ago that “evolution in perfusion over the last 50 years from its development was minimal due to several reasons, most of them financial or from lack of incentive for upgrading the technology” (2). Nonetheless, it prophetically emphasized the potential role that the mini-CPB might play in this field. Obviously, the contemporary technology of MiECC corresponds to this vision. Evolution of mini-CPB over the last 10 years led to the foundation and establishment of Minimal invasive Extracorporeal Technologies International Society (MiECTiS), which defined the MiECC system over dispersed custom-made mini-CPB designs and managed to put order in the chaos of its abundant as well as arbitrary terminology (3). Nowadays, MiECC is a specified technology that integrates all contemporary advancements in perfusion science by comprising certain components: a closed circuit with biologically inert blood contact surfaces and reduced priming volume; a centrifugal pump; a membrane oxygenator; a heat exchanger; a venous bubble trap or venous air removing device; a cardioplegia system; a shed blood management device. A major achievement of MiECC systems throughout the last decade was their evolution from type I to type IV design. Currently, a 4th generation hybrid modular MiECC system (integrating a hard-shell venous reservoir as a stand-by component for immediate conversion to an open system) overcomes all safety concerns and all unexpected intraoperative scenarios in the cardiac OR (4), and hence it is compatible with all cardiac surgical case-mix (Figure 1).
Clinical evidence of MiECC superiority
The major advantage of MiECC systems intrinsic characteristics is that they abet best applying cardiovascular physiology to intraoperative perfusion, while they ultimately unify all three operative disciplinary techniques into a common strategy; this joined holistic effect of MiECC may be considered as a therapy in cardiovascular diseases (5). In the present era of evidence-based medicine, current clinical evidence justifies, literally unanimously, the superiority of MiECC over conventional CPB (cCPB) in reducing haemodilution and better preserving haematocrit, thus in reducing the need for perioperative blood transfusion (3). These results forced recently the EACTS/EACTA Task Force to integrate MiECC as an intraoperative strategy for maintenance of haemostasis and blood conservation management in adult cardiac surgery (6). Moreover, MiECC significantly reduces the incidence of postoperative atrial fibrillation and improves renal and myocardial protection (class IA), attenuates systemic inflammatory response, reduces cerebral gaseous microembolization and preserves end-organ function (class IIB) (3).
The largest relevant meta-analysis which includes 2,700 patients from 24 randomized controlled trials (RCTs) proved that MiECC was associated not only with reduced postoperative morbidity, but also with less mortality in CABG as compared to cCPB (0.5% vs. 1.7%; P=0.02) (7). As shown in Table 2, the observed clinical benefits from MiECC use become more evident as more patients are included in the study. The same pattern applies for the RCTs comparing MiECC versus cCPB, as evidenced by Merkle et al. (12). This strong favourable effect of MiECC was found to all large-scale analyses. Thus, a well-designed propensity score analysis including 3,139 patients undergoing elective CABG further established these favourable results (30-day mortality: 0.8% for MiECC vs. 2.7% for cCPB; P<0.001) (13). Moreover, the large network meta-analysis performed including 22,778 patients showed that MiECC significantly reduced 30-day all-cause mortality from CABG compared to cCPB and off-pump CABG (OPCAB) (1.20% for MiECC vs. 1.94% for OPCAB vs. 2.59% for cCPB) (14). Regarding the comparison of MiECC to OPCAB in this meta-analysis, even though no significant statistical differences were demonstrated, the superiority of MiECC was evidenced by the hierarchy of treatments in the probability analysis; this ranked MiECC as the first treatment followed by OPCAB and cCPB (14). Thus, MiECC should not represent just a compromise between OPCAB and cCPB as concluded in this meta-analysis (14), but an attractive dominant technique in coronary surgery (15). These results are consistent with all subsequent available meta-analyses (16,17).

Full table
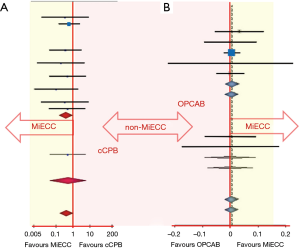
Criticism on MiECC clinical superiority supports that results from meta-analyses of mostly underpowered RCTs are biased, speculative and do not reflect the real-world. However, there is a strong element which renders results from the literature fair. This is the consistency of the outcomes from each one of the RCTs which are included to the meta-analyses, comparing either MiECC with cCPB or MiECC with OPCAB. This is schematically represented in Figure 2, where it is obvious that results from major RCTs are all located on the same side of the statistical axis favouring MiECC, while none favours the opposite. Correspondingly, this is the common pattern of every single paper published in the literature, which compares MiECC with any other perfusion technique. Thus, the consistency of these results locks the reliability and validity of MiECC superiority.
However, in order to overcome criticism, there is an urgent need for a new, large, high quality RCT to definitively address the emerging effectiveness of MiECC. For this, COMICS trial was designed. This is a multi-centre RCT powered to investigate the effectiveness and cost-effectiveness of using MiECC in all patients operated for CABG, AVR or CABG+AVR. COMICS was launched in May 2018 and it is expected to recruit 3,500 patients randomized in two cohorts (MiECC and cCPB) from four continents and as far as 30 centres. The trial was planned so as to overcome most limitations of previous RCTs dealing with MiECC; it evaluates MiECC systems that meet pre-specified MiECTiS criteria and it is adequately powered to influence clinical practice and prevent bias.
Rationale for MiECC use
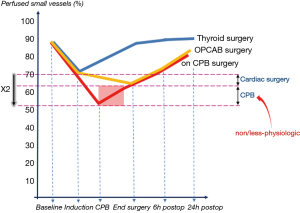
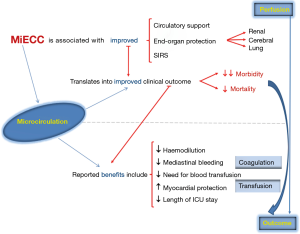
As far as the rationale of MiECC superior results is concerned, it has been demonstrated that these stem from the best applied physiology in perfusion. This is because MiECC is a closed system that allows optimal perfusion with higher mean arterial pressure for any given flow as well as with systemic vascular resistance close to normal values (18); consequently, there is reduced requirement for vasoactive drugs (19). In cellular level, contemporary research indicates that the main favourable result from MiECC use lies in the integrity of microcirculation. It is well known that cardiac surgery, with or without CPB use, is inevitably associated with an inflammatory reaction that promotes microcirculatory alterations (20). As evidenced in Figure 3, the proportion of perfused small vessels decreases significantly after induction of anesthesia in the CPB group as compared to OPCAB patients; it slightly improves thereafter surgery and it returns to baseline almost 24 hours after cardiac surgery. It seems then that the CPB use nearly doubles the detrimental effect of cardiac surgery itself on microcirculation and renders any procedure as non/less physiologic. On the other hand, optimal perfusion during MiECC is primarily attributed to significantly reduced haemodilution and microcirculatory hypoperfusion as compared to cCPB (21). Moreover, MiECC enhances recovery of microcirculatory blood flow leading to faster restoration of nutritive microvascular blood flow (22). In overall, this observed integrity of microcirculation when operated on MiECC translates into improved end-organ protection and may explain most of its clinical benefits (Figure 4).
Towards a “more physiologic” perfusion
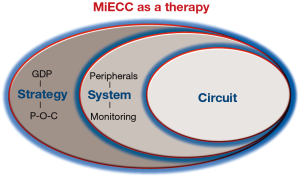
Cardiac surgery represents a “non-physiologic” intervention since it perturbs the physiologic milieu in many aspects. We have recently introduced in the literature the concept of a “more physiologic” cardiac surgery in order to emphasize the need for further improvement of patients’ outcomes (5). This policy proclaims a multidisciplinary perioperative strategy based on goal-directed perfusion (GDP) while incorporating thorough in-line monitoring and continuous intraoperative regulation of offered treatment. This translates to a “prevent rather than correct” perfusion policy, through real-time adjustment rather than correction of any derangement detected late by incremental evaluation. Such a strategy upgrades MiECC from just a CPB circuit to a system, and then to a multidisciplinary procedure, which involves and unifies all three stakeholders of the surgical team so as to obtain the maximum benefit from all (3). Thus, this multidisciplinary perioperative strategy for attaining “more physiologic” cardiac surgery literally advances MiECC from a circuit to therapy (Figure 5): the circuit is the base, the peripherals (autotransfusion device, in-line monitoring sets, anaesthetic protocols, transoesophageal echocardiography, etc.) upgrade this to a system, while a multidisciplinary strategy encompasses all and renders MiECC use a holistic approach to cardiac surgery. Such a strategy encounters surgeon’s and anaesthesiologist’s particular technique, implementation of GDP from perfusionist’s perspective and point-of-care (POC) heparin/protamine and coagulation management from the anaesthesiologist’s perspective (5). The ultimate goal of such a policy when using MiECC is to operate on high-risk patients and to perform complex procedures as comfortable, in terms of haemodynamic and metabolic integrity, as operating on a low-risk case (23). This is because a “more physiologic” intraoperative perfusion is of paramount importance in this particular setting for optimizing outcome.
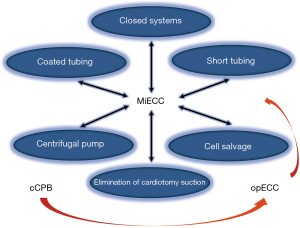
From cCPB to opECC prior to MiECC
MiECC has been developed in the past decade as the best contemporary perfusion technology available in clinical practice. After establishment of MiECC, the term “optimized ECC” (opECC) has been introduced by some surgical teams, confirming the need for shifting from cCBP towards a better perfusion circuit (i.e., with short tubing, biocompatible surfaces, centrifugal pumps, low-prime oxygenators, assisted venous drainage) (Figure 6) (24). This term refers to integration of some of the CPB technological advancements in the common perfusion practice and hence upgrading the cCPB. Its introduction highlights the breakthrough that MiECC offered to perfusion by changing mindsets and striking perfusionists to improve cCPB technology. As evidenced by the recent study of Ariyaratnam et al. (25), any opECC is just a custom-made low-grade circuit design, which will never reach the efficiency of a MiECC system neither will implement the concept of MiECC therapy we advocate.
Conclusions
Taking under account the available clinical and research data from the literature, the need for reduced morbidity in cardiac surgery and the contemporary quest for “more physiologic” intraoperative perfusion, we consider that MiECC represents the state-of-the-art in perfusion. Therefore, we advocate that MiECC should become the standard practice in performing cardiac surgery.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- D'Agostino RS, Jacobs JP, Badhwar V, et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 Update on Outcomes and Quality. Ann Thorac Surg 2018;105:15-23. [Crossref] [PubMed]
- Brian BF. The engineering of cardiopulmonary bypass. In: Mongero LB, Beck JR. editors. On bypass: Advanced perfusion techniques. New Jersey: Humana Press Inc, 2008:1-28.
- Anastasiadis K, Murkin J, Antonitsis P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 2016;22:647-62. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Argiriadou H, et al. Modular minimally invasive extracorporeal circulation systems; can they become the standard practice for performing cardiac surgery? Perfusion 2015;30:195-200. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Deliopoulos A, et al. A multidisciplinary perioperative strategy for attaining "more physiologic" cardiac surgery. Perfusion 2017;32:446-53. [Crossref] [PubMed]
- Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:79-111. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Haidich AB, et al. Use of minimal extracorporeal circulation improves outcome after heart surgery; a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol 2013;164:158-69. [Crossref] [PubMed]
- Benedetto U, Angeloni E, Refice S, et al. Is minimized extracorporeal circulation effective to reduce the need for red blood cell transfusion in coronary artery bypass grafting? Meta-analysis of randomized controlled trials. J Thorac Cardiovasc Surg 2009;138:1450-3. [Crossref] [PubMed]
- Biancari F, Rimpilainen R. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart 2009;95:964-9. [Crossref] [PubMed]
- Zangrillo A, Garozzo FA, Biondi-Zoccai G, et al. Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: a meta-analysis of randomized controlled studies. J Thorac Cardiovasc Surg 2010;139:1162-9. [Crossref] [PubMed]
- Harling L, Warren OJ, Martin A, et al. Do miniaturized extracorporeal circuits confer significant clinical benefit without compromising safety? A meta-analysis of randomized controlled trials. ASAIO J 2011;57:141-51. [Crossref] [PubMed]
- Merkle F, Haupt B, El-Essawi A, et al. State of the art in cardiovascular perfusion: now and in the next decade. HSR Proc Intensive Care Cardiovasc Anesth 2012;4:211-6. [PubMed]
- Ried M, Kobuch R, Rupprecht L, et al. Reduced 30-day mortality in men after elective coronary artery bypass surgery with minimized extracorporeal circulation - a propensity score analysis. BMC Cardiovascular Disorders 2012;12:17. [Crossref] [PubMed]
- Kowalewski M, Pawliszak W, Raffa GM, et al. Safety and efficacy of miniaturized extracorporeal circulation when compared with off-pump and conventional coronary artery bypass grafting: evidence synthesis from a comprehensive Bayesian-framework network meta-analysis of 134 randomized controlled trials involving 22 778 patients. Eur J Cardiothorac Surg 2016;49:1428-40. [Crossref] [PubMed]
- Anastasiadis K, Antonitsis P, Bauer A, et al. Minimal invasive Extracorporeal Technologies international Society (MiECTiS). Minimal invasive extracorporeal circulation should become the standard practice in coronary revascularization surgery. Eur J Cardiothorac Surg 2016;50:189. [Crossref] [PubMed]
- Benedetto U, Ng C, Frati G, et al. Miniaturized extracorporeal circulation versus off-pump coronary artery bypass grafting: a meta-analysis of randomized controlled trials. Int J Surg 2015;14:96-104. [Crossref] [PubMed]
- Winkler B, Heinisch PP, Gahl B, et al. Minimally invasive extracorporeal circulation circuit is not inferior to off-pump coronary artery bypass grafting: Meta-analysis using the Bayesian method. Ann Thorac Surg 2017;103:342-50. [Crossref] [PubMed]
- Wiesenack C, Liebold A, Philipp A, et al. Four years' experience with a miniaturized extracorporeal circulation system and its influence on clinical outcome. Artif Organs 2004;28:1082-8. [Crossref] [PubMed]
- Bauer A, Diez C, Schubel J, et al. Evaluation of hemodynamic and regional tissue perfusion effects of minimized extracorporeal circulation (MECC). J Extra Corpor Technol 2010;42:30-9. [PubMed]
- De Backer D, Dubois MJ, Schmartz D, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. Ann Thorac Surg 2009;88:1396-403. [Crossref] [PubMed]
- Yuruk K, Bezemer R, Euser M, et al. The effects of conventional extracorporeal circulation versus miniaturized extracorporeal circulation on microcirculation during cardiopulmonary bypass-assisted coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg 2012;15:364-70. [Crossref] [PubMed]
- Donndorf P, Kuhn F, Vollmar B, et al. Comparing microvascular alterations during minimal extracorporeal circulation and conventional cardiopulmonary bypass in coronary artery bypass graft surgery: a prospective, randomized study. J Thorac Cardiovasc Surg 2012;144:677-83. [Crossref] [PubMed]
- Asteriou C, Antonitsis P, Argiriadou H, et al. Minimal extracorporeal circulation reduces the incidence of postoperative major adverse events after elective coronary artery bypass grafting in high-risk patients. A single-institutional prospective randomized study. Perfusion 2013;28:350-6. [Crossref] [PubMed]
- Ganushchak YM, Körver EP, Yamamoto Y, et al. Versatile minimized system - a step towards safe perfusion. Perfusion 2016;31:295-9. [Crossref] [PubMed]
- Ariyaratnam P, Mclean LA, Cale A, et al. Mini-extracorporeal circulation technology, conventional bypass and prime displacement in isolated coronary and aortic valve surgery: a propensity-matched in-hospital and survival analysis. Interact Cardiovasc Thorac Surg 2018;27:13-9. [Crossref] [PubMed]


