
If you hear hoofbeats, sometimes it is actually a zebra: tumor characteristics and presentation, rather than age, should dictate assessment of esophageal neoplasms
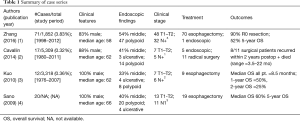
Esophageal sarcomatoid carcinoma (ESC), first described by Virchow in 1864, is a rare neoplasm which contains both a carcinomatous epithelial and sarcomatous stromal component. Comprising 0.3–2.8% of esophageal malignancies, its description in the literature is predominantly composed of individual patient reports and small case series spanning large time intervals (1-6). Most commonly, ESC presents as a polypoidal lesion of the submucosa with sarcoma within the protruding tumor mass and carcinoma at the base (3). Review of the largest series to date suggests that ESC is a predominantly male disease most often diagnosed after age 60 in the middle esophagus, optimally treated with resection often with poor prognosis (Table 1).
Full table
In the setting of low incidence and variable presentation, the case report, “A young man with progressive esophageal neoplasms,” by Shen et al. highlights the inherent challenges associated with the diagnosis and management of ESC, but more importantly, allows for comment on evaluation of esophageal neoplasms in young patients. In this report, a 23-year-old male presented with 4 months of symptoms including chest pain and reflux. The patient underwent an additional 4 months of assessment and biopsy, which demonstrated spindle cell histology with inconclusive malignant behavior. Following international consultation with 6 experts, a positron emission tomography/computed tomography (PET-CT) scan was obtained which demonstrated multiple systemic metastases consistent with advanced disease. The authors concluded the case by reporting that surgery was avoided because of metastatic disease; however, it is unclear how much time passed from the first symptoms to the end of the expert consultation and whether a delay occurred. Furthermore, the diagnosis of ESC was never confirmed.
The discussion by the expert panel and this patient’s history provide an important basis for highlighting the need for more aggressive evaluation of young patients with highly suspicious tumors. In unusual presentations of disease, it is important to consider the entire clinical picture and history of the patient rather than focusing on the piece that does not fit when evaluating for a potential malignancy. Although a recent series reported a median diagnostic age in the 6th decade of life, Zhang et al. supported early studies demonstrating wider variability in age by showing that over 60% of patients in their series were younger than 60 (range, 26–82); however, the patient in this report is certainly much younger than typically observed across all series, which may explain the delay in oncologic assessment in the setting of a suspicious clinical presentation (1-4).
Most reports of ESC describe rapidly progressive dysphagia as the predominant presenting symptom, rather than reflux or chest pain as in this case (1,5,6). However, in the current case the patient was noted to have atypical reflux symptoms with EGD demonstrating ulceration and “white moss” with pathology diagnosed as submucosal spindle cell hyperplasia. No further work-up was done at this time to further differentiate whether the lesion was benign or malignant. In the subsequent 2 months he was noted to be refractory to PPI, had bloody phlegm and thickened esophageal walls with a narrowed lumen on CT—all findings that are suspicious for neoplasm that should have warranted a more aggressive evaluation. At the time of the patient’s next EGD, 4 months later, the lesion had already had a 6-fold increase in size with endoscopic ultrasound demonstrating involvement of the mucosa and submucosal layers and pathology with fusiform cell tumor. By the time the patient underwent further oncologic staging with a PET-CT scan, the tumor had undergone an 8-fold increase in size, the patient had immunohistochemistry (IHC) suggestive of ESC now with multiple sites of distant disease on imaging. Perhaps there was a missed opportunity for cure in this patient if surgery had been done early in this highly suspicious process.
ESC predominantly appears as a large intraluminal polypoid mass on esophagogastric-duodenoscopy (EGD), with rapid growth (2–5 months doubling time) contributing to the early onset and rapid progression of obstructive symptoms (3,5,7). Ulceration, as in this case, has been described in 12.5–33% of patients (3). Previous histologic analyses have suggested that the polypoidal nature of ESC may reflect early independent growth by the sarcomatous and carcinomatous components of the tumor, with the sarcoma forming the protruding tumor mass (3,8). In cases where the carcinomatous component is dominant, the disease may present as an ulceration. Most frequently, the carcinoma portion is a squamous cell carcinoma; however, cases of adenocarcinoma or neuroendocrine disease have been described (1,3).
It is unclear from this case report how much time elapsed, and whether the 3-year survival refers to time since the initial symptoms versus completion of the staging PET scan. If it is the latter, it is possible that ESC may be a misdiagnosis given that previous studies have described a high rate of nodal metastasis and poor prognosis associated with this disease (1,2). Alternative diagnoses such as granular cell tumor or sarcoidosis could be considered. In a patient with a progressively growing esophageal lesion, think cancer until proven otherwise regardless of age. Although we are not told the elapsed time between symptoms and subsequent staging PET scan, if this patient did have broadly metastatic ESC then we have missed a potential opportunity for curative intervention. At this time, given the extensive disease, time will quickly tell if this is truly ESC.
This case highlights multiple missed opportunities for earlier and more aggressive staging and management, including endoscopic or surgical resection. Given the clinical course, it appears several warning signs were not heeded, and the tumor subsequently grew at a rapid rate. This possibly reflects a broader general view of esophageal neoplasms as a disease of the elderly. Unfortunately, this perception may explain studies demonstrating later stage diagnoses in younger patients (9,10). When presented with a young patient with an atypical history and a rapidly growing lesion, it is important for physicians to remember that sometimes Occam’s razor does not hold true.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhang B, Xiao Q, Yang D, et al. Spindle cell carcinoma of the esophagus: A multicenter analysis in comparison with typical squamous cell carcinoma. Medicine (Baltimore) 2016;95:e4768. [Crossref] [PubMed]
- Cavallin F, Scarpa M, Alfieri R, et al. Esophageal carcinosarcoma: management and prognosis at a single Italian series. Anticancer Res 2014;34:7455-9. [PubMed]
- Kuo CJ, Lin TN, Lin CJ, et al. Clinical manifestation of esophageal carcinosarcoma: a Taiwan experience. Dis Esophagus 2010;23:122-7. [Crossref] [PubMed]
- Sano A, Sakurai S, Kato H, et al. Clinicopathological and immunohistochemical characteristics of esophageal carcinosarcoma. Anticancer Res 2009;29:3375-80. [PubMed]
- Madan AK, Long AE, Weldon CB, et al. Esophageal carcinosarcoma. J Gastrointest Surg 2001;5:414-7. [Crossref] [PubMed]
- Zhao S, Xue Q, Ye B, et al. Synchronous primary carcinosarcoma and adenosquamous carcinoma of the esophagus. Ann Thorac Surg 2011;91:926-8. [Crossref] [PubMed]
- Sasajima K, Taniguchi Y, Morino K, et al. Rapid growth of a pseudosarcoma of the esophagus. J Clin Gastroenterol 1988;10:533-6. [Crossref] [PubMed]
- Chino O, Kijima H, Shimada H, et al. Clinicopathological studies of esophageal carcinosarcoma: analyses of its morphological characteristics using endoscopic, histological, and immunohistochemical procedures. Endoscopy 2000;32:706-11. [Crossref] [PubMed]
- Portale G, Peters JH, Hsieh CC, et al. Esophageal adenocarcinoma in patients < or = 50 years old: delayed diagnosis and advanced disease at presentation. Am Surg 2004;70:954-8. [PubMed]
- Hashemi N, Loren D, DiMarino AJ, et al. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Dig Dis Sci 2009;54:1708-12. [Crossref] [PubMed]


