
Video-assisted thoracoscopic surgery in trauma: pros and cons
Introduction
Thoracic injury accounts for 60% of all trauma cases (1). Injuries may range from simple rib fractures to complex cardiac or tracheobronchial injuries. Appropriate understanding of the mechanism of injury and astute clinical examination will highlight the majority of thoracic injuries. If thoracic injury is suspected appropriate investigation, most commonly by computerised tomography (CT) scan should be carried out within the emergency department. CT scan can determine between intrapleural blood, contusion, atelectasis and intra-parenchymal haemorrhage where chest radiography fails.
Most thoracic injuries (85%) are managed conservatively with analgesia, respiratory support and physiotherapy. This will encompass most simple rib fractures and small lung contusions. Within this group, the majority (85%) of haemothoraces are managed by tube thoracostomy (2). Guidelines recommend referral for surgery for acute blood loss over 1,500 mL or recorded ongoing drainage of more than 200–400 mL over 2 to 4 hours (3). Fifteen percent of patients with thoracic trauma will require surgical intervention either due to haemorrhage, disruption of an intrathoracic organ requiring repair or reconstruction.
Thoracoscopic surgery has become increasingly popular since the early nineties for a wide range of general thoracic surgical procedures. Although the first recorded use of video-assisted thoracoscopic surgery (VATS) in trauma goes back to 1946 (4). There seems to be a paucity in literature over the established use of VATS in traumatic injuries of the thorax. Early adopters of VATS in thoracic injuries determine its use by type and location of injury, patient characteristics and surgeon experience.
Management of haemothoraces
The majority of patients requiring thoracic surgery for trauma will be for assessment and evacuation of haemothoraces (48–62%) and the management of small vessel bleeding (5,6). The basic principle is to assess the extent of intra-thoracic injury, arrest bleeding and drain the haemothorax. Blood within the pleural space is a good medium for bacterial growth and delayed drainage increases the risk of intrapleural infections.
There is evidence in the literature that VATS, in well selected patients, is superior to tube thoracostomy and leads to decreased post-traumatic infection (17.8% vs. 46.5%, P=0.004) length of ventilatory dependency (7.2 vs. 13.6 days, P=0.015) and overall hospital stay (19.4 vs. 34.1 days, P=0.001) (7). While there was a difference in mortality, this was not statistically different in this study. What remains the main challenge is the correct timing of thoracoscopic drainage of haemothoraces (5,8,9). It transpires that VATS and wash out in the first 5 days post-trauma offers shorter duration of chest tube drainage, length of stay and post-trauma infections. Close observation should be carried out for those with traumatic haemothoraces who are treated with thoracostomy tubes. Failure to observe a “clean” pleural space within the first 24 to 72 hours following tube thoracostomy, despite vigorous physiotherapy and mobilisation to those trauma patients who can, VATS should be strongly considered (10). VATS washouts applied after the first 5 days risk the presence of early adhesions, converting the plain thoracoscopy into a complex one, increasing the risk of parenchymal injury with air leaks and the development of empyema.
For patients undergoing VATS surgery, single lung ventilation is ideal, allowing adequate visualisation. If the patient is unable to tolerate this due to underlying lung pathology or significant contusion, double lung ventilation with intermittent apnoea is an option. A well-trained thoracic anaesthetist is a valid investment in such cases to provide adequate ventilation with low tidal volumes, if part of the procedure needs to be done with two lung ventilation. Alternative options, in case of inadequate oxygenation with single lung ventilation, include jet ventilation through a flexible bronchoscope or a cook exchange catheter directed to the main bronchus of the operated lung. Patients should be placed in the lateral decubitus position with flexing of the table to allow widening of the intercostal spaces. With current evidence, single or multiport approaches are equally acceptable. Following adequate drainage and washout of the hemithorax a chest drain should be placed prior to closure. It is vital to ensure the lung fully expands to occupy the space. If there is residual space blood can recollect and lead to post-operative complications. The reader should remember that injured lungs have a compromised compliance and the presence of low suction might be advisable to promote drainage of pleural fluid.
Lung lacerations
Lung lacerations were classified in 1998 based on the mechanism of injury and the associated CT findings (11). Type III lacerations are caused by punctures from adjacent rib fractures (12). These tend to be peripheral and thus appropriate for surgical management. Repairs are justified by ongoing blood loss or air leak through the thoracostomy tube. The development of stapling devices makes VATS an appropriate method of management. Twenty-six percent of thoracic trauma cases may require wedge resection for lung lacerations with the use of VATS (5). It is important to note the volume of lung loss and careful consideration should be taken with regards to whether this should be conservatively managed or resected. Patient factors play a vital role in this decision process. Patients with known history of pulmonary pathology and limited respiratory reserves might restrict the surgeon’s ability to resect large volumes of lung. In the case of deep penetrating lung injuries and excessive blood loss an emergency thoracotomy is usually the procedure of choice. If stability is relatively present the choice of VATS depends on the experience of the surgeon, the hospital set up and the pathology to be attended. Patients who require an anatomical lung resection or attention of deep parenchymal injuries, were single ventilation is not guaranteed should not be managed with VATS. Where minimally invasive procedures are possible the overall execution of surgery should take in consideration the injury, the position of injury, the overall condition of the patient, associated injuries and the length of required anaesthetic.
Diaphragmatic injury
Diaphragmatic injuries account for 3% of all trauma cases (13). The mechanism of diaphragmatic injury is often high impact injuries which lead to a sudden increase in intra-abdominal pressure causing rupture. The right diaphragm is protected by the liver; hence, 80% of ruptures occur on the left. High level of suspicion needs to be kept in these types of injuries as CT has a low sensitivity of 53–74% (14). Chest radiography can also be elusive with 50% of initial radiographs being normal (Figures 1,2) (13). This can also present as a pneumothorax leading to thoracostomy tubes inserted into hollow abdominal organs. This is one of the reasons that diaphragmatic injuries can present late. Other reasons include delayed rupture of a devitalised diaphragmatic muscle and expansion of an originally small tear (15). The role of VATS in this situation is invaluable as it has a 100% sensitivity and allows for repair at the same time (16).
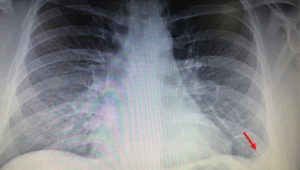
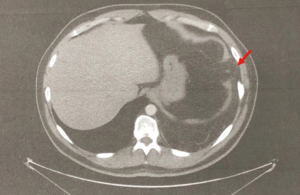
With the increase in elective VATS diaphragmatic repairs surgeons are becoming more accustomed to repairing the diaphragm (17). This is also attributed to the availability of endoscopic suturing devices. VATS approach holds an additional advantage to abdominal approaches, such as laparoscopy, as it allows for better visualisation to the posterior recess (2). In addition to the surgical considerations discussed above, the patient should be placed into the Trendelenburg position to facilitate adequate visualisation of the diaphragm. Nasogastric tube placement is important to deflate the stomach and can facilitate reduction of any herniation. Simple interrupted sutures should be enough to repair the majority of acute defects (18). Mesh repairs should be reserved for chronic or large defects.
Oesophageal injury
Traumatic oesophageal injuries are rare although, when occur, have a high mortality rate. For those who receive surgical intervention, within the first 24 hours, the published mortality is 13% (19). This raises to 55% for those identified and treated outside that period. The pathophysiology of oesophageal trauma is often linked to barotrauma. Intra-luminal pressures of 5 psi are sufficient to cause perforation (20). Eighty percent of ruptures are along the left posterolateral wall, 2 to 6 centimetres above the diaphragm (19). The remaining happen on the posterior or right posterolateral wall. Suspicion of injury should be aroused by retrosternal or epigastric pain, the presence of pleural effusions, subcutaneous emphysema, mediastinal air or persistent pyrexia. Pleural effusions occur after breach of the mediastinal pleura. Absence of an effusion can delay the diagnosis.
Following early diagnosis surgery should not be delayed beyond 24 hours, as the likelihood of post-repair leak increases. A conservative approach, with antibiotics and nasogastric aspiration, almost always leads to treatment failure and subsequent need for thoracotomy for empyema. Traditionally upper and middle third perforations are approached via thoracotomy via the right 4th or 5th intercostal space. Lower third perforations are best approached via the left 6th or 7th space. The traditional management of this syndrome is to carry out a buttressed two-layer repair of the oesophagus. This will require an open approach.
Limited studies have demonstrated equivalent outcomes in those who were treated after the first 24 hours by VATS drainage of the mediastinum (21). The notion is to limit the surgical insult, especially given the high rate of post-repair leaks. Exposure of the mediastinum was carried out from the diaphragm to the arch and an irrigation catheter placed in the mediastinum at the site of the perforation. Chest drain allowed for passive drainage. This group kept a low threshold for conversion if access was insufficient and thus had a 20% conversion rate. Approach was made on the side the leak was thought to have originated from. It is important to note that patients require early nutrition via a naso-jejunal route and thoracic irrigation should be initiated following VATS mediastinal debridement. A subsequent case report discussed the management of a delayed presentation of an oesophageal rupture. This group used a VATS approach with mediastinal and pleural debridement and insertion of T-tube through the site of perforation (22). While this is a single case report these studies provide promise in the use of VATS for oesophageal injuries. The need for thoracotomy in oesophageal perforation still carries a key role in early presentation, access is difficult, adequate drainage of the mediastinum is not guaranteed and there is associated trapped lung requiring a complex decortication.
Traumatic chylothorax
Traumatic chylothorax is extremely rare in the setting of trauma. This can occur through penetrating or blunt trauma. Presentation is often delayed and noted subsequently by respiratory distress and a pleural effusion (23). Mortality in postoperative cases is 24% however in the setting of non-iatrogenic trauma this rises to 50% (23,24). This is most likely attributed to a delayed diagnosis and management. That being said, with the appropriate conservative management traumatic causes are more likely to resolve as compared with non-traumatic causes (50% vs. 27%, P=0.048) (25).
The initial management of this condition is the placement of a thoracoscopy tube to allow drainage, assessment of volume drained and confirmation of diagnosis through presence of triglycerides (>110 mg/dL) chylomicrons. A low-fat diet or parenteral nutrition will aim to reduce the flow of lymph. A volume of greater than 1,000 mL/day or a leak of greater than 100 mL/day for 2 weeks is likely to suggest failure of conservative management and should prompt surgical intervention (26). Surgical treatment in the setting of traumatic duct injury is likely to resolve the chylothorax quickly and efficiently (25,26).
The use of VATS ligation of the thoracic duct is well described (27). A left lateral decubitus position is utilised with flexion and some anterior rotation to allow access to the right posterior mediastinum. The inferior pulmonary ligament is freed to allow reflection of the lower lobe anteriorly. Dissection of the mediastinal pleura between the azygous vein and the oesophagus will allow for gentle retraction of the oesophagus anteriorly. If the duct is identified it can be clipped and divided or a stapler can be used for this process. If it is not identified all fatty and lymphatic tissue between the azygous vein, posteriorly, the oesophagus, anteriorly and the aorta/right pleura distally can be divided. The VATS approach is especially useful in this setting as it provides a 10-fold increase in magnification and the 30-degree scope allows for visualisation between the spine and the aorta where the thoracic duct can reside (27).
Tracheobronchial injuries
Tracheobronchial injuries following trauma are extremely rare. Given that the majority of patients with such injuries do not make it to the hospital the true incidence of this condition is not known. Autopsy reports suggest 2.5–3.2% of patients who die from trauma have tracheobronchial injuries (28). Eighty percent of such injuries are due to blunt trauma and are located within 2.5 cm of the carina (29). Without prompt diagnosis this condition is fatal. Presentation occurs with respiratory distress, surgical emphysema, pneumothorax and a large air leak following insertion of a chest tube. The airway should be secured without delay and diagnostic bronchoscopy performed. If the injury is distal to the carina, selective ventilation of the unaffected side will aid with ventilation and subsequent repair. Given the urgency of this situation and the challenges faced with ventilation during the repair an open approach is advocated.
A trans cervical approach with the additional use of VATS has been described, however, this was a small distal laceration that could have been managed with conservative selective ventilation (30). Similarly, VATS approach to tracheal resection and reconstruction has been described but this was in an elective setting related to a tracheal neoplasm (31). This allows time for appropriate investigation, anaesthetic and surgical planning; often not possible in the emergency setting.
Contraindications to VATS
The benefits of VATS in elective thoracic surgery is well documented. Decreased pain, pulmonary complications, prolonged air leak, arrhythmias, hospital stay, and post-operative quality of life are all improved by VATS surgery (32,33). The benefits of decreased pain, pulmonary complications, hospital stay and quality of life have been translated into the use of VATS in the trauma setting (8,34). In addition to these benefits, 50% of those undergoing VATS for suspected diaphragm injuries are negative; reducing the rate of negative thoracotomies (35).
Relative contra-indications for the use of VATS in the trauma setting include previous thoracic surgery, previous pleurodesis or radiological signs of dense adhesions. In addition to this a low threshold to convert to thoracotomy should be kept if access is challenging. Lung contusions are likely to be present in the setting of thoracic trauma. Depending on the extent of difficulty ventilating, VATS should be considered a relative contraindication. As discussed, the presence of tracheobronchial injury is a challenging emergency. It possesses challenges not only to the surgeon but also to the anaesthetic team and thus should not be considered for VATS. Furthermore, haemodynamically unstable patients should be resuscitated aggressively, and an open approach will allow rapid control of haemorrhage.
Key points
Thoracic trauma carries a high mortality. The burden of injury often extends beyond the thoracic cavity. In many situations, consideration to the surgical approach should be made to limit the surgical insult. The majority of patients can be managed with thoracostomy tubes however close observation should be continued to assess for failure of treatment. VATS should be considered early for those who have evidence of ongoing bleeding, retained haemothorax or air leaks beyond 72 hours or sooner with evidence of insufficient lung expansion in the presence of a large air leak. Those presenting with evidence of oesophageal injury should be treated by thoracotomy if within the first 24 hours otherwise VATS approach for debriding the mediastinum and setup of irrigation is demonstrated to be equivalent to an open approach. Patients with traumatic chylothorax should be given the opportunity of conservative management given the rate of response to this approach. If treatment fails VATS approach can provide superior visualisation of the duct.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mahoozi HR, Volmerig J, Hecker E. Modern management of traumatic hemothorax. J Trauma Treat 2016;5:326. [Crossref]
- Yuh DD, Doty JR, Vricella LA, et al. Thoracic Trauma. In: Yuh DD, Vricella LA, editors. John Hopkins Textbook of Cardiothoracic Surgery. Second Edi. New York, NY: McGraw Hill Education; 2014. p. 9-24.
- ATLS Subcommittee; American College of Surgeons’ Committee on Trauma; International ATLS working group. Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg 2013;74:1363-6.
- Martins Castello Branco J. Thoracoscopy as a method of exploration in penetrating injuries of the thorax. Dis Chest 1946;12:330-5. [Crossref] [PubMed]
- Goodman M, Lewis J, Guitron J, et al. Video-assisted thoracoscopic surgery for acute thoracic trauma. J Emerg Trauma Shock 2013;6:106-9. [Crossref] [PubMed]
- Galbal A, Alghorori M. Role of emergency VATS in blunt chest trauma patients. J Cardiothorac Surg 2013;8:O73. [Crossref]
- Chou YP, Kuo LC, Soo KM, et al. The role of repairing lung lacerations during video-assisted thoracoscopic surgery evacuations for retained haemothorax caused by blunt chest trauma. Eur J Cardiothorac Surg 2014;46:107-11. [Crossref] [PubMed]
- Meyer DM, Jessen ME, Wait MA, et al. Early evacuation of traumatic retained hemothoraces using thoracoscopy: a prospective, randomized trial. Ann Thorac Surg 1997;64:1396-400. [Crossref] [PubMed]
- Lin HL, Huang WY, Yang C, et al. How early should VATS be performed for retained haemothorax in blunt chest trauma. Injury 2014;45:1359-64. [Crossref] [PubMed]
- Chou YP, Lin HL, Wu TC. Video-assisted thoracoscopic surgery for retained hemothorax in blunt chest trauma. Curr Opin Pulm Med 2015;21:393-8. [Crossref] [PubMed]
- Wagner RB, Crawford WO Jr, Schimpf PP. Classification of parenchymal injuries of the lung. Radiology 1988;167:77-82. [Crossref] [PubMed]
- Thoongsuwan N, Kanne JP, Stern EJ. Spectrum of blunt chest injuries. J Thorac Imaging 2005;20:89-97. [Crossref] [PubMed]
- Karmy-Jones R, Jurkovich G. Management of Blunt Chest and Diaphragm Injuries. In: Patterson A, Cooper JD, Deslaeuiers J, et al. editors. Pearson’s Thoracic & Esophageal Surgery. 3rd Edition. Phiadelphia, PA: Churchill Livingston Elsevier; 2008:1768-76.
- Jaroslaw K. ESTS Textbook of Thoracic Surgery. Cracow: Medycyna Praktyczna; 2015.
- Sirbu H, Busch T, Spillner J, et al. Late bilateral diaphragmatic rupture: challanging diagnostic and surgical repair. Hernia 2005;9:90-2. [Crossref] [PubMed]
- Paci M, Ferrari G, Annessi V, et al. The role of diagnostic VATS in penetrating thoracic injuries. World J Emerg Surg 2006;1:30. [Crossref] [PubMed]
- Nardini M, Jayakumar S, Elsaegh M, et al. Left video-assisted thoracoscopic surgery for hemidiaphragm traumatic rupture repair. Interact Cardiovasc Thorac Surg 2017;24:815-6. [Crossref] [PubMed]
- Parelkar SV, Oak SN, Patel JL, et al. Traumatic diaphragmatic hernia: Management by video assisted thoracoscopic repair. J Indian Assoc Pediatr Surg 2012;17:180-3. [Crossref] [PubMed]
- Fell SC. Esophageal perforation. In: Patterson G, Cooper JD, Deslauriers J, et al. editors. Pearson’s Thoracic & Esophageal Surgery. 3rd edition. Phiadelphia, PA: Churchill Livingston Elsevier; 2008:792-808.
- Mackler SA. Spontaneous rupture of the oesophagus: an experimental and clinical study. Surg Gynecol Obs 1952;93:345-56.
- Haveman JW, Nieuwenhujis VB, Kobold JPM, et al. Adequate debridement and drainage of the mediastinum using open thoracotomy or video-assisted thoracoscopic surgery for Boerhaave’s syndrome. Surg Endosc 2011;25:2492-7. [Crossref] [PubMed]
- Do YW, Lee CY, Lee S, et al. Successful management of delayed esophageal rupture with T-tube drainage using video-assisted thoracoscopic surgery. Korean J Thorac Cardiovasc Surg 2016;49:478-80. [Crossref] [PubMed]
- Dorsey JF, Morris GE. Traumatic Rupture of The Thoracic Duct With Chylothorax: A Brief Review Of The Literature. JAMA 1942;119:337-8. [Crossref]
- Shah RD, Luketich JD, Schuchert MJ. Postesophagectomy chylothorax: incidence, risk factors, and outcomes. Ann Thorac Surg 2012;93:897-903. [Crossref] [PubMed]
- Maldonado F, Cartin-Ceba R, Hawkins FJ, et al. Medical and surgical management of chylothorax and associated outcomes. Am J Med Sci 2010;339:314-8. [Crossref] [PubMed]
- Schild HH, Strassburg CP, Welz A, et al. Treatment options in patients with chylothorax. Dtsch Arztebl Int 2013;110:819-26. [PubMed]
- Devulapalli C, Anderson J, Llore NNP, et al. Thoracic duct ligation: Right video-assisted thoracoscopic surgery approach. Oper Tech Card Thorac Surg 2016;21:152-9. [Crossref]
- Bertelsen S, Howitz P. Injuries of the trachea and bronchi. Thorax 1972;27:188-94. [Crossref] [PubMed]
- Lynn RB, Iyengar K. Traumatic rupture of the bronchus. Chest 1972;61:81-3. [Crossref] [PubMed]
- Angelillo-Mackinlay T. Transcervical repair of distal membranous tracheal laceration. Ann Thorac Surg 1995;59:531-2. [Crossref] [PubMed]
- Li S, Liu J, He J, et al. Video-assisted thoracoscopic surgery resection and reconstruction of thoracic trachea in the management of tracheal neoplasm. J Thorac Dis 2016;8:600-7. [Crossref] [PubMed]
- Joshi V, Kirmani B, Zacharias J. Thoracotomy versus VATS: is there an optimal approach to treating pneumothorax?. Ann R Coll Surg Engl 2013;95:61-4. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Ahmed N, Jones D. Video-assisted thoracic surgery: state of the art in trauma care. Injury 2004;35:479-89. [Crossref] [PubMed]
- Ochsner MG, Rozycki GS, Lucente F, et al. Prospective evaluation of thoracoscopy for diagnosing diaphragmatic injury in thoracoabdominal trauma: a priliminary report. J Trauma 1993;34:704-9; discussion 709-10. [Crossref] [PubMed]


