
The role of liposomal bupivacaine in thoracic surgery
For advocates of regional analgesia, the appeal of a local anesthetic formulation with a duration of action of several days is clear. Liposomal bupivacaine (LB), now with FDA approval for local infiltration and interscalene nerve block—but not neuraxial use though it has been used there (1), provides multiple days of local anesthetic availability though at a lower tissue concentration than standard bupivacaine preparations (bupivacaine hydrochloride; BH). The process of determining the clinical situations where such a local anesthetic formulation can improve perioperative analgesia, simplify its conduct, and/or decrease costs is ongoing, and appears to be very much procedure dependent. A recent study by Dominguez et al. (2) focuses attention on perioperative analgesia for thoracic surgery and the potential contributions of LB to this unique patient population.
LB and its expanding applications
LB is actually BH encased in liposomes that steadily release bupivacaine over a prolonged period of time. LB has a potential duration of action of up to 120 hours (96 hours for local infiltration and 120 hours for interscalene block) (3). The generally accepted maximal dose is 266 mg which is available in a volume of 20 mL and is frequently diluted with saline to provide sufficient volume for the intended administration. It has been combined with BH to achieve a higher immediate tissue concentration of BH, but caution should be exercised regarding potentially toxic concentrations of BH. Combination of LB with local anesthetics other than BH will lead to release of BH from the liposomes, again leading to potentially toxic concentrations of BH and eliminating the reservoir of BH that would be released over time. Although LB has a favorable safety profile when compared to BH when injected intravascularly, intrathecally or epidurally in animals (4), surveillance data indicates that LB use does not prevent local anesthetic toxicity (5). While the duration of action of LB is much greater than BH, the tissue concentrations of local anesthetic achieved by LB are less than what would typically occur with standard BH. The implications of this were nicely demonstrated by Abildgaard et al. (6) in whose study an indwelling interscalene catheter with BH had greater analgesic efficacy and duration of action than a single interscalene injection of LB for total shoulder arthroplasty. Another consideration with LB is the approximately 100-fold cost of LB relative to BH, implicitly requiring that the cost of using LB be adequately offset by medical benefits and cost-savings from its use.
Studies seeking to exploit the duration of action of LB have been limited in their unanimity as to its analgesic benefits, and this likely reflects different study populations, methods of local anesthetic administration, outcome variables, study durations, study designs and size, and control groups across the multitude of studies currently available. From this evolving experience, it is now evident that LB cannot simply be thought of as a long-acting variant of standard BH. Rather, it appears to work well for procedures that require moderate analgesia for a longer duration but do not require a particularly dense block to achieve an acceptable analgesic level, recalling that a dense block (e.g., motor) is often neither necessary or desirable. Examples where LB is effective include hemorrhoidectomy, bunionectomy, inguinal hernia repair, and breast augmentation (7). For major abdominal surgery, Boogaerts et al. demonstrate that epidurally dosed LB can produce analgesia of longer duration without motor blockade (1), though they simply determined whether pain was endurable or not. In contrast, surgical site and intraarticular injection did not demonstrate meaningful differences in outcomes compared to placebo groups (8).
Long-lasting analgesia mediated by local anesthetics at concentrations known to be effective can often be achieved by continuous infusion of local anesthetic with some type of infusion device, typically patient-controlled. Examples include catheters placed in the epidural space, perineurally, in a suitable fascial plane, or at the incision site. Catheters certainly provide the clinician more options for postoperative pain management in that infusion rates, concentrations, and adjuvants, such as epinephrine or opioid, can generally be modified while the catheter is in place. In contrast, a potential benefit of LB is that it can provide long-term analgesia when catheter placement may not be possible or desired. Not placing a catheter avoids the added risks, costs, complexity, and effort of catheter placement, maintenance and removal that typically require a dedicated acute pain service. When catheters are used to continuously administer local anesthetic, their success requires that they not be dislodged. Further, leaving catheters in place is challenging for anticoagulated patients, risks infection and catheter retention and, for neuraxial catheters, may produce a sympathectomy and associated hemodynamic concerns. That being said, if a single administration of LB can achieve and maintain a clinically meaningful level of analgesia in the perioperative period, it could revolutionize the use of regional analgesia.
Postoperative thoracic surgery pain and evolving strategies to treat it
The motivation for focusing only on regional analgesia, and not regional anesthesia, for thoracic surgery is clear. In contrast to many other types of surgery where regional anesthesia can be utilized as the sole anesthetic and may later contribute to analgesia, regional techniques used in thoracic surgery are oriented toward perioperative analgesia since major thoracic surgery is performed only under general anesthesia. This consideration means that it is only necessary to deliver local anesthetic concentrations sufficient to produce analgesia to the effective site, something that LB may be able to achieve. The focus on analgesia, though sufficient, is also a necessity since uncontrolled postoperative pain from even minimally invasive thoracic surgery can have important consequences apart from the discomfort it produces. Patients with adequate pain control are better able to ambulate, breathe deeply, cough, and clear secretions. Effective analgesia facilitates return to preoperative activity levels, and helps prevent deep vein thrombosis, atelectasis, pneumonia, and acute to chronic pain conversion (9). Although patients undergoing procedures of the thoracic cavity with minimally invasive techniques report less acute postoperative pain (10), the incidence of acute to chronic pain conversion can still be as high as 47% (11), which is no different than the value of 50% typically quoted for open thoracotomy (9). These observations are likely due to the smaller incisions used by minimally invasive procedures but similar levels of trauma to intercostal nerves for both open and minimally invasive approaches.
Several regional techniques are used to manage pain that accompanies thoracic surgery. For several decades, but only that, thoracic epidural analgesia (TEA) has served as the analgesic standard for patients undergoing thoracic surgery. For thoracic procedures, epidural analgesia is superior to parenteral analgesia and appears to reduce complications (12,13). However, given decreased acute pain control requirements following minimally invasive approaches (10), epidural analgesia is not seen as necessary for this class of thoracic procedures as compared to thoracotomy (14). In addition, the hemodynamic lability accompanying the sympathectomy of TEA, concerns for the neuraxis related to anticoagulation, and concerns about catheter dislodgement, replacement and eventual removal motivates alternatives to TEA. Intercostal nerve blocks (ICNB) have a history that predates TEA use in thoracic surgery and, as indicated in Table 1 and the next section, the limitation of a single injection may be solved by use of LB. Unilateral paravertebral catheter placement provides appropriate coverage (22) with decreased concerns for sympathectomy, though little is known about single injections with LB. Less established in thoracic surgery are the fascial plane blocks. Erector spinae nerve blocks have also been used to provide analgesia following thoracic surgery (23). Relatively little is known about the analgesic properties of erector spinae blocks compared to other blocks used for thoracic surgery, much less their use with LB.
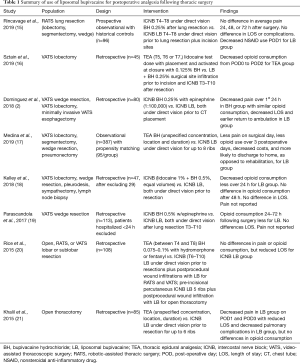
Full table
LB and thoracic surgery
Much of what is currently known about the use of LB to provide perioperative analgesia in association with thoracic surgery is summarized in Table 1, where the study of Dominguez et al. (2) is emphasized with a bold typeface. Several broad observations can be made. First, LB use in thoracic surgery has yet to be subject to the scrutiny of a randomized controlled trial. The trials summarized in Table 1 retrospectively examine outcomes following introduction of LB to thoracic surgery and draw their controls from prior to that time. Most studies were of minimally invasive procedures, particularly video-assisted thoracoscopic surgery (VATS) or robotic-assisted thoracic surgery (RATS), though one included some open thoracotomies while another included only open thoracotomies. The LB intervention was always some form of ICNB, typically under direct vision though one involved percutaneous ICNB, with considerable variation between studies as to whether the ICNB was performed prior to or after lung resection. The greatest variation was in the control group which consisted of TEA and ICNB, sometimes activated at different times relative to lung resection than for the intervention group. It is notable that for ICNB controls, the concentration of BH was generally 0.25%, with only one study (19) employing BH 0.5%, thus frequently mitigating the potential benefit of higher concentrations of BH in the control groups. However, in the one study with a higher concentration of BH in the control group, opioid consumption was still greater for the control group, though length of stay (LOS) did not differ. With the exception of one study which included percutaneous ICNB with LB for open thoracotomy, all blocks were placed after access to the thoracic cavity was achieved, though this was sometimes proceeded by local infiltration of the port sites. Finally, outcome measures varied and included combinations of pain, opioid consumption, functionality, costs, LOS and whether discharged to home or rehabilitation. Given the retrospective nature of the pain assessments, none of these studies appeared to benefit from pain assessment tools that were administered frequently, assessed the range of pain experienced, determined whether the origin of the pain was from the chest tube, from the incision, was referred, or related pain to activities such as ambulation, coughing or deep inspiration. Given the premise of many of these studies, that pain control is important because it decreases postoperative complications through improved pulmonary toilet and increased ambulation, no quantitative assessments of pulmonary function are available, only one study reports ambulation, and outcomes from any type physiologic assessment tool are not available. Considering the concerns about development of chronic post-thoracotomy pain, even following VATS and RATS, it would be desirable to have longer-term data on pain and functionality, and perhaps determine the extent that this pain is neuropathic in character. Direct or implicit cost savings are particularly important given the not inconsiderable difference in the cost of LB compared to BH.
All but one of the studies summarized in Table 1 demonstrated some benefit for the LB group. These benefits ranged from decreased nonsteroidal anti-inflammatory drug (NSAID) use on postoperative day 1 (POD1) to decreases in pulmonary complications to reductions in LOS. Four of the studies, including that of Dominguez et al. (2), report decreased LOS or greater likelihood of discharge to home. Of note, the reductions in pain or opioid consumption reported in association with decreased LOS or greater likelihood of discharge to home were not large and sometimes nonexistent. However, relationships between pain and physical activity following thoracotomy are complex and not intuitive (24). Certainly, a reduction in pulmonary complications and improved ambulation are consistent with reduced LOS. One concern about reported reductions in LOS is that all studies summarized in Table 1 are retrospective in nature and report differences following LB’s introduction into practice. Therefore, the reported benefits may simply reflect evolving institutional protocols, even for ambulation and care following discharge. Nonetheless, the use of LB could certainly be the reason for these positive outcomes which occurred within LB’s established duration of action.
The study of Dominguez et al. (2) is 1 of at least 4 recent studies that have compared ICNB with BH and LB. Comparing ICNB with BH 0.25% to ICNB with LB, both placed under direct vision prior to chest tube placement, they demonstrated decreased LOS and improved ambulation in the BH group. While the BH group reported decreased levels of pain, there were no differences in opioid consumption. The decreased LOS in the LB group is consistent with the earlier return of ambulation in the LB group. While seemingly inconsistent, the decreased pain reported by the BH group in their study was hypothesized by them to represent a consequence of improved ambulation in the LB group, perhaps demonstrating a need for more discriminating pain assessments in future studies. As pointed out earlier, the relationships between pain and physical activity following at least open thoracotomy are not always direct (24). Though opioid consumption did not differ between the two groups, it is only a surrogate marker and the fact that important benefits were achieved without differences is notable. Importantly, there was not even a trend toward greater readmission or emergency room visits for those in the LB group despite the greater rate of earlier discharge. Although it is a retrospective design, the study of Dominguez et al. (2) identified in advance and reported LOS as its primary outcome. Further and in contrast to many other potentially relevant outcomes discussed previously, LOS is an outcome that can be accurately assessed even from a retrospective vantage point.
Future goals for regional analgesia in thoracic surgery
Ideally, for thoracic surgery, a long-acting local anesthetic would provide effective analgesia with a single injection, outlast the expected duration of acute pain and, through this, eliminate drawbacks associated with continuous catheter techniques. Further, likely in combination with suitable pharmacologic adjuncts, a single injection of a long-acting anesthetic could permit a transition to an opioid-free analgesic regimen, thereby limiting many of the side effects and concerns of opioid use. Short of that, it should permit a direct transition to an oral opioid regimen without an intermediate period requiring intravenous opioids. On a broader time scale, an effective regional adjunct for thoracic surgery of all types should limit the development of post-thoracotomy pain syndrome. Although it may seem improbable that a relatively brief intervention can have implications months or even years afterward, studies with aggressive perioperative use of TEA have reported some of the lowest rates of post-thoracotomy pain (9,24,25). Consequently, well-conducted TEA remains the standard insofar as pain relief following thoracic surgery is concerned. However, TEA retains all of the limitations of catheter-based techniques and the added risks of proximity to the neuraxis.
The current state of the literature appears to support LB rather than BH 0.25% for ICNB placed under direct vision during minimally invasive approaches to the thoracic cavity. There may be some suggestion that ICNB with LB performs favorably compared to TEA for both open and minimally invasive approaches, although many details regarding TEA in those studies are not provided (Table 1). Clearly, conclusions based on the retrospective set of observations contained in Table 1 must undergo prospective evaluation with more detailed and long-term assessments of pain, physical function and cost. Moreover, TEA and ICNB are not the only routes for the adjunctive administration of local anesthetics in conjunction with thoracic surgery. Paravertebral techniques have already been extensively studied for thoracic surgery (26), and erector spinae approaches (23) may offer additional benefits, though little is known about the use of either with LB. Finally, an appropriate milieu of systemic adjuncts such as the gabapentinoids (27) may be necessary to achieve any of the broad goals enumerated above.
Conclusions
The work of Dominguez et al. (2) adds to and is somewhat consistent with a recent set of observational studies (Table 1) that appear to support the use of LB for ICNB in place of BH, and suggest benefits for ICNB with LB even when compared to TEA. Collectively, these studies are sufficient to motivate additional and more detailed prospective evaluations of LB while continuing to explore other routes for providing regional analgesia in association with thoracic surgery. What continues to be encouraging is how far we have come in the past few decades.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Boogaerts JG, Lafont ND, Declercq AG, et al. Epidural administration of liposome-associated bupivacaine for the management of postsurgical pain: a first study. J Clin Anesth 1994;6:315-20. [Crossref] [PubMed]
- Dominguez DA, Ely S, Bach C, et al. Impact of intercostal nerve blocks using liposomal versus standard bupivacaine on length of stay in minimally invasive thoracic surgery patients. J Thorac Dis 2018;10:6873-9. [Crossref] [PubMed]
- Exparel [package insert]. Pacira Pharmaceuticals, Inc: San Diego, CA 92121 USA; November, 2018. Available online: https://www.exparel.com/hcp/prescriptioninformation.pdf. Accessed April 8, 2019.
- Joshi GP, Patou G, Kharitonov V. The safety of liposome bupivacaine following various routes of administration in animals. J Pain Res 2015;8:781-9. [Crossref] [PubMed]
- Aggarwal N. Local anesthetics systemic toxicity association with exparel (bupivacaine liposome)- a pharmacovigilance evaluation. Expert Opin Drug Saf 2018;17:581-7. [Crossref] [PubMed]
- Abildgaard JT, Lonergan KT, Tolan SJ, et al. Liposomal bupivacaine versus indwelling interscalene nerve block for postoperative pain control in shoulder arthroplasty: a prospective randomized controlled trial. J Shoulder Elbow Surg 2017;26:1175-81. [Crossref] [PubMed]
- Pozek JP, Beausang D, Segna KG, et al. Controlled-Release Local Anesthetics. In: Hadzic A. editor. Hadzic's Textbook of Regional Anesthesia and Acute Pain Management. 2nd edition. New York: McGraw-Hill Education; 2017:138-46.
- Malik O, Kaye AD, Kaye A, et al. Emerging roles of liposomal bupivacaine in anesthesia practice. J Anaesthesiol Clin Pharmacol 2017;33:151-6. [PubMed]
- Gottschalk A, Cohen SP, Yang S, et al. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594-600. [Crossref] [PubMed]
- Förster R, Storck M, Schäfer JR, et al. Thoracoscopy versus thoracotomy: a prospective comparison of trauma and quality of life. Langenbecks Arch Surg 2002;387:32-6. [Crossref] [PubMed]
- Steegers MA, Snik DM, Verhagen AF, et al. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain 2008;9:955-61. [Crossref] [PubMed]
- Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA 2003;290:2455-63. [Crossref] [PubMed]
- Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ 2000;321:1493. [Crossref] [PubMed]
- Kaplowitz J, Papadakos PJ. Acute pain management for video-assisted thoracoscopic surgery: an update. J Cardiothorac Vasc Anesth 2012;26:312-21. [Crossref] [PubMed]
- Rincavage M, Hammond L, Reddy S, et al. Pain control using liposomal bupivacaine versus bupivacaine for robotic assisted thoracic surgery. Int J Clin Pharm 2019;41:258-63. [Crossref] [PubMed]
- Sztain JF, Gabriel RA, Said ET. Thoracic epidurals are associated with decreased opioid consumption compared to surgical infiltration of liposomal bupivacaine following video-assisted thoracoscopic surgery for lobectomy: a retrospective cohort analysis. J Cardiothorac Vasc Anesth 2019;33:694-8. [Crossref] [PubMed]
- Medina M, Foiles SR, Francois M, et al. Comparison of cost and outcomes in patients receiving thoracic epidural versus liposomal bupivacaine for video-assisted thoracoscopic pulmonary resection. Am J Surg 2019;217:520-4. [Crossref] [PubMed]
- Kelley TM Jr, Bailey DW, Sparks P, et al. Intercostal Nerve Blockade with Exparel® Results in Lower Opioid Usage during the First 24 Hours after Video-Assisted Thorascopic Surgery. Am Surg 2018;84:1433-8. [PubMed]
- Parascandola SA, Ibañez J, Keir G, et al. Liposomal bupivacaine versus bupivacaine/epinephrine after video-assisted thoracoscopic wedge resection†. Interact Cardiovasc Thorac Surg 2017;24:925-30. [Crossref] [PubMed]
- Rice DC, Cata JP, Mena GE, et al. Posterior intercostal nerve block with liposomal bupivacaine: an alternative to thoracic epidural analgesia. Ann Thorac Surg 2015;99:1953-60. [Crossref] [PubMed]
- Khalil KG, Boutrous ML, Irani AD, et al. Operative intercostal nerve blocks with long-acting bupivacaine liposome for pain control after thoracotomy. Ann Thorac Surg 2015;100:2013-8. [Crossref] [PubMed]
- Fibla JJ, Molins L, Mier JM, et al. The efficacy of paravertebral block using a catheter technique for postoperative analgesia in thoracoscopic surgery: a randomized trial. Eur J Cardiothorac Surg 2011;40:907-11. [PubMed]
- Forero M, Rajarathinam M, Adhikary S, et al. Continuous Erector Spinae Plane Block for Rescue Analgesia in Thoracotomy After Epidural Failure: A Case Report. A A Case Rep 2017;8:254-6. [Crossref] [PubMed]
- Ochroch EA, Gottschalk A, Augostides J, et al. Long-term pain and activity during recovery from major thoracotomy using thoracic epidural analgesia. Anesthesiology 2002;97:1234-44. [Crossref] [PubMed]
- Gottschalk A, Ochroch EA. Clinical and demographic characteristics of patients with chronic pain after major thoracotomy. Clin J Pain 2008;24:708-16. [Crossref] [PubMed]
- Hill SE, Keller RA, Stafford-Smith M, et al. Efficacy of single-dose, multilevel paravertebral nerve blockade for analgesia after thoracoscopic procedures. Anesthesiology 2006;104:1047-53. [Crossref] [PubMed]
- Solak O, Metin M, Esme H, et al. Effectiveness of gabapentin in the treatment of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2007;32:9-12. [Crossref] [PubMed]


