Treatment strategy and decision-making for elderly surgical candidates with early lung cancer
Introduction
The general population in many developed countries is aging. According to the Japanese national statistics on population in 2017 (1), average life expectancy was 81.1 years in men and 87.3 years in women. The median residual life expectancy at age 80 years was 9.0 years in men and 11.8 years in women. Non-small cell lung cancer (NSCLC) is the most common malignancy and is a disease of the elderly.
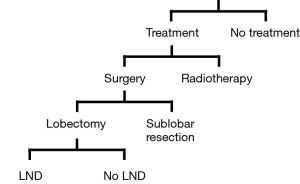
The current standard treatment of early stage NSCLC is lobectomy plus mediastinal lymph node dissection, regardless of lymph node involvement. This procedure is feasible and safe even in octogenarians, given the appropriate selection of surgical candidates (2). According to the evidence-based clinical practice guidelines, in elderly patients who are potential candidates for curative surgical resection, surgery should not be dismissed based solely on chronologic age (3). However, if the patient’s general status is impaired, other treatment options, such as lobectomy without lymph node dissection, sublobar resection, and radiotherapy would be considered (Figure 1). For a subset of the patients, no radical treatment may be offered because of the patient’s poor condition. When treating elderly patients with lung cancer, patients’ conditions, such as comorbid diseases and functional impairment due to aging, are a significant concern. Decision-making before surgery must therefore carefully balance the risks and benefits from the short- and long-term perspectives.
The purpose of this review article is to provide an overview of the options and to compare the treatment outcomes reported in the literature.
Treatment patterns
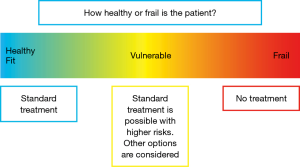
Among elderly cancer patients of the same chronological age, there is a wide range of heterogeneity in physical, mental, and social conditions. When an elderly patient comes to a thoracic surgeon, the surgeon will first consider how healthy the patient is. If the patient looks healthy or in fit condition, the surgeon offers standard treatment. On the other hand, if the patient looks particularly frail, the surgeon may not offer any kind of treatment (Figure 2). In a clinical practice, a substantial percentage of elderly patients are in a vulnerable condition. They are neither 100% healthy nor very frail. For such patients, standard treatment is possible, but there are higher risks.
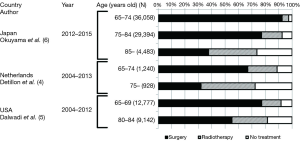
Treatment patterns for elderly patients with stage I NSCLC have been reported (4-10). The percentages of surgery, radiotherapy, and no treatment were different among the different age groups (Figure 3). According to the Japanese hospital-based cancer registry, the majority of younger patients received surgery alone, while a greater proportion of patients aged 85 years or over received radiation therapy (34.9%) or did not receive any cancer treatment (25.4%) (6). This age-dependent treatment pattern was similarly observed in other reports of the population-based database (4,5). In the report from the Netherlands, the study period was divided into two halves, and the treatment pattern was compared between the first half [2004–2008] and the second half [2009–2013] (4). The use of surgery remained constant, while that of radiotherapy increased in the second half, and fewer patients received neither treatment over the years. From these data, patients with early stage lung cancer received different treatments based on their age, and older patients were less likely to receive surgery.
If the patients do not receive any cancer treatment, their prognosis is miserable (11-13). It has been demonstrated that the median overall survival of patients with untreated early stage NSCLC ranges from 9 to 14 months. The majority of these patients died of progressive cancer resulting in metastatic disease or respiratory compromise rather than comorbid conditions. Nanda et al. compared outcomes in patients older than 70 years with early stage NSCLC between stereotactic radiotherapy (SBRT) and no treatment with data from the national cancer database. Untreated patients had a poorer median survival of 10.1 months, compared with 29 months for patients who had been treated with SBRT (P<0.001) (11). This survival benefit of SBRT was consistently observed across all age groups, including those patients aged 85 years and older. The authors concluded that the use of SBRT for the treatment of elderly patients with comorbid conditions was supported.
In summary, treatment patterns vary according to the patients’ age, and patients of highly advanced age with stage I NSCLC are less likely to receive surgery than their younger counterparts.
Surgery or radiotherapy
Surgery offers the highest probability of cure in early stage NSCLC. However, surgeons often hesitate to recommend pulmonary resection for elderly patients because of the higher perioperative risks and the uncertain long-term benefit. For such patients, radiotherapy is an alternative option. According to the guidelines, for patients with comorbidities or other reasons for inoperability, presenting with a peripherally located stage I NSCLC, or any patient refusing surgery, SBRT is the preferred treatment (14).
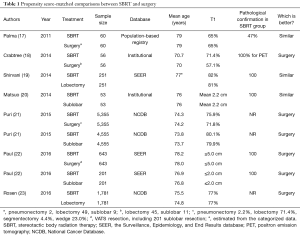
These modalities have been compared in two randomized controlled trials, but they were terminated early before completion of the study due to poor patient accruals. Although a pooled analysis of them was published (15), the total number of evaluable patients for both studies was very low, and the conclusion has not been well accepted (16). For this reason, propensity score-matched comparisons between SBRT and surgery are examined in this section (Table 1).
Full table
Palma et al. compared overall survival after surgery versus SBRT in elderly patients (75 or older) with stage I NSCLC from the North-Holland population-based registry (17). The mean age of both cohorts was 79 years. The 3-year overall survival (OS) was 42% for SBRT and 60% for surgery, and the difference was not significant (P=0.22). The authors concluded that similar OS outcomes are achieved with surgery or SBRT. Another comparison of the Surveillance, Epidemiology, and End Results database (SEER) in the USA of elderly patients (median age of the overall cohort: 75 years) demonstrated similar overall survival and lung cancer-specific survival (CSS) at 3 years for lobectomy and SBRT (HR: 1.01, P=0.94 for OS and HR: 1.00, P=0.99 for CSS) (19). According to an institutional retrospective comparison between SBRT and sublobar resection (SLR) from Japan, the differences in OS and cancer-specific death rates were both not significant (5-year OS 40.4% for SBRT and 55.6% for SLR, P=0.124; 5-year CSS 35.3% for SBRT and 30.3% for SLR, P=0.427) for smaller-sized tumors (mean tumor size ≤2.2 cm) in patients at high risk for lobectomy (20). These three studies were all characterized by advanced patients’ age.
Four matched-pair comparisons reported from surgeons are also listed in Table 1. A national population-based, retrospective cohort study from the SEER database compared CSS and OS after thoracoscopic resection and SBRT for elderly patients (≥66 years) according to tumor size (22). In the analysis of patients with smaller tumors (≤2.0 cm), overall survival was better in the surgery group (HR: 1.80, P<0.001; 52.2% for SBRT and 68.4% for sublobar surgery at 3 years), and CSS was similar in both groups (HR: 1.32, P=0.32; 82.6% for SBRT and 86.4% for sublobar surgery at 3 years). For tumors sized ≤5.0 cm, OS and CSS were both better in the surgery group (HR: 1.92, P<0.001 for OS and 2.10, P<0.001 for CSS). Crabtree et al. collected 56 matched pairs from among 458 surgical patients and 151 SBRT patients in their institution and showed better OS and disease-free survival in the surgical group (3-year OS 52% for SBRT and 69% for surgery, P=0.05; 3-year DFS 47% for SBRT and 65% for surgery, P=0.01) (18). Puri et al. collected 5,355 pairs of SBRT versus surgery and 4,555 pairs of SBRT versus sublobar resection using a propensity scoring method from the national cancer database in the USA (21). They demonstrated that median survival was longer in the surgical group than in the corresponding SBRT group (62.3 months for surgery vs. 33.1 months for SBRT, P<0.001). A similar result was obtained comparing SLR and SBRT (48.3 months for sublobar resection vs. 33.9 months for SBRT, P<0.001). Rosen et al. (23) demonstrated a striking difference in OS between SBRT and lobectomy in healthy patients with stage I lung cancer (5-year survival 59% for lobectomy vs. 29% for SBRT P<0.001, and median survival 71 months for lobectomy and 39 months for SBRT).
In summary, among seven reports, four reports showed survival advantages with surgical treatment, and three showed similar long-term outcomes. With older patient age, the difference in OS between surgery and SBRT seems to be less.
Lobectomy or sublobar resection
Lobectomy has been the standard of care for NSCLC, except adenocarcinoma in situ or minimally invasive cancer, since the Lung Cancer Study Group demonstrated a threefold increase of local recurrence and a tendency toward decreased survival among patients who underwent sublobar resection (24). Sublobar resection such as wedge resection and segmentectomy could be indicated in patients with stage I NSCLC, who may tolerate operative intervention but not a lobar resection because of comorbid disease or decreased cardiopulmonary function. Since cardiopulmonary function decreases with age, elderly patients may benefit from pulmonary parenchyma-preserving surgery in compensation for the risk of local recurrence. In fact, sublobar resection was performed in one-third (33.2%) of stage I NSCLC in cases in octogenarians (25).
In this context, several retrospective studies supported sublobar resection as an alternative for elderly patients. According to the data from the National Cancer registry in the USA, although lobectomy confers significant survival benefit over limited resection in younger patients, the significant difference in survival between lobectomy and sublobar resection disappeared at 71 years of age and the older population (26).
There are several reports comparing overall survival between lobectomy and sublobar resection in elderly patients with stage I NSCLC in a retrospective manner (Table 2). Four institutional and one multicenter report demonstrated equivalent overall survivals between two surgical procedures for elderly patients (27,28,30-32). On the other hand, two propensity score-matching comparisons of SEER demonstrated better long-term survival after lobectomy than that after sublobar resection (19,29). Shirvani et al. showed that sublobar resection was associated with worse OS and CSS than lobectomy in elderly patients (66 years or older) (19). In another matched analysis, the long-term outcomes were compared in patients age ≥65 years with small sized tumors (≤2.0 cm) between lobectomy and sublobar resection according to tumor histology (29). In patients with adenocarcinoma, segmentectomy was equivalent to lobectomy, but wedge resection was inferior to lobectomy in terms of overall survival. In patients with squamous cell carcinoma, wedge resection or segmentectomy was not equivalent to lobectomy.
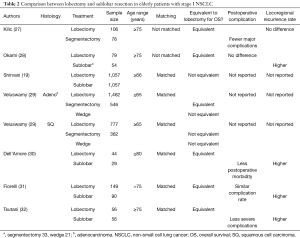
Full table
In addition to the long-term outcomes, postoperative complications are another serious concern when treating elderly patients. Tsutani and others found that lobectomy was an independent predictive factor for postoperative complications (27,30,32). Okami et al. and Kilic et al. reported that the occurrence of complications after sublobar resection was similar to that after lobectomy in a report of an unmatched cohort (28,31). This was explained by the fact that patients undergoing sublobar resection as a less invasive option had more comorbid diseases than patients undergoing lobectomy. As seen in the previous randomized trial, locoregional recurrence was more frequently observed in patients after sublobar resection (28,30-32). Notably, when the locoregional recurrence rate after sublobar resection was compared between segmentectomy and wedge resection, the rate was significantly higher in patients with wedge resection (31).
In summary, sublobar resection seems to become an alternative option for elderly patients (≥75 years). Segmentectomy may have the advantage over wedge resection of reducing locoregional recurrence.
Lymph node dissection
Although evidence for the survival benefit of mediastinal lymph node dissection has not been reported, this procedure is routinely performed with lobectomy as a standard of care. Even if 18F-fluorodeoxy glucose-positron emission tomography (FDG-PET) is used, preoperative nodal staging is not the same as the pathological staging after surgery. It is known that occult or unexpected lymph node metastasis is found in 5–15% of patients with preoperative N0 disease. Therefore, lymph node dissection is essential to achieve the highest probability of microscopic complete resection. In addition, if nodal metastasis is found by lymph node dissection, patients may have an opportunity to receive adjuvant chemotherapy, which provides a modest survival benefit (33).
Mediastinal lymph node dissection is generally a small surgical procedure. It requires an additional 15–30 min of operative time and 5–20 mL of blood loss if uneventful. However, for elderly patients, if the patients are thought to be at higher risk for lobectomy, surgeons would consider skipping this procedure to minimize surgical intervention. According to the EORTC (European Organization for Research and Treatment of Cancer) taskforce recommendation, omission of mediastinal lymph node dissection is proposed (34). There are several reports regarding this issue, and the references are classified into the Pros and the Cons of this proposal in Table 3. Three institutional reports demonstrated no survival benefit of lymph node dissection in elderly patients (35-37). Chida et al. also observed the increased number of postoperative cardiac complications in patients after mediastinal lymph node dissection (35). From a nationwide analysis of patients aged ≥80 years with stage I NSCLC, lymph node dissection was identified as one of the risk factors for postoperative complications on multivariate analysis (28). From the surgical point of view, recurrent laryngeal nerve exposure, devascularization of the bronchial wall, and increased surgical exudate or bleeding due to lymph node dissection are thought to be associated with several possible complications.
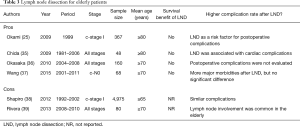
Full table
Rivera et al. demonstrated that unexpected lymph node metastasis in preoperative N0 disease was more common in the elderly population than in their younger counterpart (39). Shapiro et al. compared perioperative mortality and morbidity in elderly patients (≥65 years) with stage I NSCLC after extensive (>10 nodes) lymph node dissection or limited (≤10 nodes) lymph node dissection (38). Since the results were similar in the two groups, they concluded that extensive lymph node dissection is safe, without compromising postoperative recovery.
A couple of key points summarize the discussion. First, omission of mediastinal lymph node dissection can be considered an option after accurate preoperative staging because this procedure may increase operative risks in elderly patients. Second, if the patients cannot tolerate evidence-based adjuvant chemotherapy, the benefit of lymph node dissection will be limited.
Pre-operative risk assessment and post-treatment outcomes
To decide the optimal treatment strategy, the preoperative assessment of elderly patients is a critical process. There are several reports identifying risk factors for surgery in elderly patients. Recent publications and risk factors are listed in Table 4. Impaired pulmonary function, higher age, male sex, comorbidities, type of surgery, and advanced lung cancer (stage or tumor size) are common factors.

Full table
The only prospective study demonstrated that the comprehensive geriatric assessment (CGA) score was one of the significant factors affecting postoperative complications (40). The CGA7 measures the level of functional independence in the following 7 categories: greeting, bathing, transportation, continence, cognition, short-term memory, and mood. It is widely used as a tool for identifying problems in daily life and achieving a holistic medical approach in older adults. Of these factors, short-term memory was identified as the most important factor. To evaluate severity of comorbidities, Charlson Comorbidity Index (CCI) was used in this study. After the statistical analysis, diabetes mellitus is identified as the most important factor in the comorbid diseases. Following the risk analyses, the authors developed a simplified risk scoring system to predict severe postoperative complications. Based on the scoring system, 19.7% and 3.0% of surgical patients ≥80 years were classified into the low-risk (<5%) group and the high-risk (≥25%) group for severe complications, respectively. A prospective validation of this system is awaited. Detillon et al. analyzed the Dutch Lung Surgery Audit database to determine the postoperative outcomes of lung cancer resections and compared the results among three age groups: ≥80, 70–79, and 60–69 years (41). They identified pulmonary function and extensive resection as the most significant factors in the highest age group. Postoperative mortality in octogenarians was 6.0% and age ≥80 years was an independent predictor of operative mortality. They suggested that octogenarians could benefit from limited resection if oncologically justified.
Overall survival is the gold standard to evaluate postoperative outcomes in the general population. However, is it always true for elderly patients, because their life expectancy is obviously limited, even though it has been extended in the population statistics? Assuring a better and comfortable life may be more important than trying to cure the disease with radical treatment or to extend biological survival under poor conditions. The use of quality of life (QOL) assessments has been introduced to evaluate postoperative outcomes. Numerous publications have been found in the literature. Among them, the QOL studies in elderly patients are listed in Table 5.

Full table
QOL in elderly patients after lobectomy was investigated by Burfeind et al. (46). The QOL was compared between the younger population (<70 years) and the older population (≥70 years). They demonstrated significant decreases in QOL at 3 months after surgery, but many of the factors returned to baseline at 6 to 12 months. There was no significant difference between age groups (<70 or ≤70 years). Ferguson et al. and Salati et al. also demonstrated similar recovery patterns after surgery regardless of patient age groups (47,48). Additionally, it was found that QOL recovery after surgery was associated with % predicted forced expiratory volume in the first second. On the other hand, a study from Germany demonstrated that elderly patients (≥70 years) after lobectomy or bi-lobectomy showed a decreased tendency to achieve the preoperative level of QOL compared to younger patients (50). Balduyck et al. prospectively evaluated QOL after lobectomy or pneumonectomy in elderly patients (70–79 years) and found that both resections had a major impact on physical functioning and dyspnea status. Furthermore, lobectomy patients have a better evolution in QOL compared to pneumonectomy patients (49).
To the best of my knowledge, there are no reports of a direct comparison of QOL after treatment in elderly patients between surgery and radiotherapy or between lobectomy and sublobar resection.
Future perspectives
A definitive decision-making process has not been established. In real-world clinical practice, a treatment decision is a complex process that depends on multiple factors, with both objective and subjective judgments made by physicians and surgeons on an individual basis (51). Patients’ major concerns regarding possible outcomes of pulmonary resection are oxygen dependence, restrictions in ambulation, and limitations in activities of daily living (ADL) in their remaining lives, not transient postoperative complications (52). In this context, to make a better decision, a well-designed observational cohort study to identify predictors of poor ADL and QOL after different treatments is needed.
Shared decision-making is a process in which a physician and patient work together to make a decision. The majority of patients had a strong desire to participate in treatment decision-making rather than delegate decisions. Mokhles et al. introduced this system for treatment selection in early-stage NSCLC and demonstrated that patients found it important to be involved in treatment decision-making (53).
Based on the objective data and patients’ preferences, a better decision can be made.
Limitations
Most of the studies introduced in this review article were performed in a retrospective fashion. In addition, the endpoints of the studies were overall survival and/or complication rates. We need to pay careful attention to interpret the studies and compare the results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Ministry of Health, Labor and welfare Japan. National Population Statistics. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/life/life17/index.html, Accessed 5 February 2019.
- Ito H, Nakayama H, Yamada K, et al. Outcomes of lobectomy in 'active' octogenarians with clinical stage I non-small-cell lung cancer. Ann Thorac Cardiovasc Surg 2015;21:24-30. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-e190S.
- Detillon D, Driessen EJM, Aarts MJ, et al. Changes in treatment patterns and survival in elderly patients with stage I non-small-cell lung cancer with the introduction of stereotactic body radiotherapy and video-assisted thoracic surgery. Eur J Cancer 2018;101:30-37. [Crossref] [PubMed]
- Dalwadi SM, Szeja SS, Bernicker EH, et al. Practice Patterns and Outcomes in Elderly Stage I Non-Small-cell Lung Cancer: A 2004 to 2012 SEER Analysis. Clin Lung Cancer 2018;19:e269-e276. [Crossref] [PubMed]
- Okuyama A, Higashi T. Patterns of cancer treatment in different age groups in Japan: an analysis of hospital-based cancer registry data, 2012-2015. Jpn J Clin Oncol 2018;48:417-25. [Crossref] [PubMed]
- Haasbeek CJ, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol 2012;23:2743-7. [Crossref] [PubMed]
- Lee K, Kim HO, Choi HK, et al. Real-world treatment patterns for patients 80 years and older with early lung cancer: a nationwide claims study. BMC Pulm Med 2018;18:127. [Crossref] [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6. [Crossref] [PubMed]
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Nanda RH, Liu Y, Gillespie TW, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: A National Cancer Data Base analysis. Cancer 2015;121:4222-30. [Crossref] [PubMed]
- Chadha AS, Ganti AK, Sohi JS, et al. Survival in untreated early stage non-small cell lung cancer. Anticancer Res 2005;25:3517-20. [PubMed]
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Meyers BF, Puri V, Broderick SR, et al. Lobectomy versus stereotactic body radiotherapy for stage I non-small cell lung cancer: Post hoc analysis dressed up as level-1 evidence? J Thorac Cardiovasc Surg 2015;150:468-71. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [Crossref] [PubMed]
- Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014;147:1183-91. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer 2014;50:2932-8. [Crossref] [PubMed]
- Puri V, Crabtree TD, Bell JM, et al. Treatment Outcomes in Stage I Lung Cancer: A Comparison of Surgery and Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1776-84. [Crossref] [PubMed]
- Paul S, Lee PC, Mao J, et al. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ 2016;354:i3570. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- Okami J, Higashiyama M, Asamura H, et al. Pulmonary resection in patients aged 80 years or over with clinical stage I non-small cell lung cancer: prognostic factors for overall survival and risk factors for postoperative complications. J Thorac Oncol 2009;4:1247-53. [Crossref] [PubMed]
- Mery CM, Pappas AN, Bueno R, et al. Similar long-term survival of elderly patients with non-small cell lung cancer treated with lobectomy or wedge resection within the surveillance, epidemiology, and end results database. Chest 2005;128:237-45. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6. [Crossref] [PubMed]
- Okami J, Ito Y, Higashiyama M, et al. Sublobar resection provides an equivalent survival after lobectomy in elderly patients with early lung cancer. Ann Thorac Surg 2010;90:1651-6. [Crossref] [PubMed]
- Veluswamy RR, Ezer N, Mhango G, et al. Limited Resection Versus Lobectomy for Older Patients With Early-Stage Lung Cancer: Impact of Histology. J Clin Oncol 2015;33:3447-53. [Crossref] [PubMed]
- Dell'Amore A, Monteverde M, Martucci N, et al. Lobar and sub-lobar lung resection in octogenarians with early stage non-small cell lung cancer: factors affecting surgical outcomes and long-term results. Gen Thorac Cardiovasc Surg 2015;63:222-30. [Crossref] [PubMed]
- Fiorelli A, Caronia FP, Daddi N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today 2016;46:1370-82. [Crossref] [PubMed]
- Tsutani Y, Tsubokawa N, Ito M, et al. Postoperative complications and prognosis after lobar resection versus sublobar resection in elderly patients with clinical Stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Pallis AG, Gridelli C, Wedding U, et al. Management of elderly patients with NSCLC; updated expert's opinion paper: EORTC Elderly Task Force, Lung Cancer Group and International Society for Geriatric Oncology. Ann Oncol 2014;25:1270-83. [Crossref] [PubMed]
- Chida M, Minowa M, Karube Y, et al. Worsened long-term outcomes and postoperative complications in octogenarians with lung cancer following mediastinal lymph-node dissection. Interact Cardiovasc Thorac Surg 2009;8:89-92. [Crossref] [PubMed]
- Okasaka T, Usami N, Taniguchi T, et al. Can non-performance of radical systematic mediastinal lymphadenectomy be justified in elderly lung cancer patients? An evaluation using propensity-based survival analysis. Eur J Cardiothorac Surg 2010;38:27-33. [Crossref] [PubMed]
- Wang Y, Wu N, Chen J, et al. Is radical mediastinal lymphadenectomy necessary for elderly patients with clinical N-negative non-small-cell lung cancer? A single center matched-pair study. J Surg Res 2015;193:435-41. [Crossref] [PubMed]
- Shapiro M, Mhango G, Kates M, et al. Extent of lymph node resection does not increase perioperative morbidity and mortality after surgery for stage I lung cancer in the elderly. Eur J Surg Oncol 2012;38:516-22. [Crossref] [PubMed]
- Rivera C, Falcoz PE, Rami-Porta R, et al. Mediastinal lymphadenectomy in elderly patients with non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;44:88-92. [Crossref] [PubMed]
- Saji H, Ueno T, Nakamura H, et al. A proposal for a comprehensive risk scoring system for predicting postoperative complications in octogenarian patients with medically operable lung cancer: JACS1303. Eur J Cardiothorac Surg 2018;53:835-41. [Crossref] [PubMed]
- Detillon DD, Veen EJ. Postoperative Outcome After Pulmonary Surgery for Non-Small Cell Lung Cancer in Elderly Patients. Ann Thorac Surg 2018;105:287-93. [Crossref] [PubMed]
- Zhang R, Kyriss T, Dippon J, et al. American Society of Anesthesiologists physical status facilitates risk stratification of elderly patients undergoing thoracoscopic lobectomy. Eur J Cardiothorac Surg 2018;53:973-9. [Crossref] [PubMed]
- Rueth NM, Parsons HM, Habermann EB, et al. Surgical treatment of lung cancer: predicting postoperative morbidity in the elderly population. J Thorac Cardiovasc Surg 2012;143:1314-23. [Crossref] [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. A model for morbidity after lung resection in octogenarians. Eur J Cardiothorac Surg 2011;39:989-94. [Crossref] [PubMed]
- Hino H, Karasaki T, Yoshida Y, et al. Risk factors for postoperative complications and long-term survival in lung cancer patients older than 80 years. Eur J Cardiothorac Surg 2018;53:980-6. [Crossref] [PubMed]
- Burfeind WR Jr, Tong BC, O'Branski E, et al. Quality of life outcomes are equivalent after lobectomy in the elderly. J Thorac Cardiovasc Surg 2008;136:597-604. [Crossref] [PubMed]
- Ferguson MK, Parma CM, Celauro AD, et al. Quality of life and mood in older patients after major lung resection. Ann Thorac Surg 2009;87:1007-12. [Crossref] [PubMed]
- Salati M, Brunelli A, Xiume F, et al. Quality of life in the elderly after major lung resection for lung cancer. Interact Cardiovasc Thorac Surg 2009;8:79-83. [Crossref] [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardiothorac Surg 2009;35:1070-5. [Crossref] [PubMed]
- Schulte T, Schniewind B, Walter J, et al. Age-related impairment of quality of life after lung resection for non-small cell lung cancer. Lung Cancer 2010;68:115-20. [Crossref] [PubMed]
- Brunelli A, Pompili C, Salati M. Patient selection for operation: the complex balance between information and intuition. J Thorac Dis 2013;5:8-11. [PubMed]
- Cykert S, Kissling G, Hansen CJ. Patient preferences regarding possible outcomes of lung resection: what outcomes should preoperative evaluations target? Chest 2000;117:1551-9. [Crossref] [PubMed]
- Mokhles S, Nuyttens J, de Mol M, et al. Treatment selection of early stage non-small cell lung cancer: the role of the patient in clinical decision making. BMC Cancer 2018;18:79. [Crossref] [PubMed]