Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model
Introduction
During the last five decades, the etiology, epidemiology, pathogenesis, and treatment of acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), which are lethal clinical conditions, have been actively studied. The recent treatment outcomes have mainly been improved with advances in intensive care strategies at the intensive care unit (ICU) and developments of life-maintaining devices. Patients with ARDS account for 10–15% of all ICU patients, of whom 80% need an artificial ventilator. The mortality of ARDS is approximately 30–60%, but treatment options for ARDS rely predominantly on increasing gas exchange and preventing complications (1). Established studies on ALI/ARDS have demonstrated that ALI/ARDS is organ failure from overactivation of the immune system which is initiated by excessive production of proinflammatory cytokines. Lung injury activates alveolar macrophages, which secret proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1α (IL-1α), and IL-6 (2). These proinflammatory cytokines increase recruitment and infiltration of inflammatory cells, especially neutrophils, into damaged tissue; activated neutrophils secret various toxic materials, including oxygen free radicals, proteases, arachidonic acid metabolites, and platelet-activating factors, which result in damage to capillary and alveolar endothelia. Then, the alveolar-capillary barrier loses the ability to regulate osmolality; in turn, proteins leak to the interstitium and induce edema, and flow into the alveolar space. Consequently, gas exchange function is disturbed, pulmonary compliance is reduced, and pulmonary artery pressure is increased (3). Based on these study results, there have been numerous studies on inhibition of proinflammatory cytokine secretion, blockage of neutrophil influx, and suppression of mediator activity. To date, neutrophil elastase inhibitors, antioxidants, and numerous proinflammatory cytokine inhibitors have been reported; however, they are still not accepted as treatment modalities in clinical practice (4). Glycyrrhizin (GL) is an extract derived from Glycyrrhiza glavra (licorice) and has been studied since the 1950s. GL has recently been reported to have various pharmacological effects like anti-inflammatory, anticarcinogenic, hepatoprotective, and antiviral activities. A recent study has demonstrated that GL has an anti-inflammatory effect on ALI; however, the pharmacological activity of GL has not yet been completely elucidated. Therefore, this study was conducted to investigate the therapeutic effect of GL on lipopolysaccharide (LPS)-induced ALI in a mouse model and to elucidate its possible mechanisms involved (5).
Methods
Experimental materials
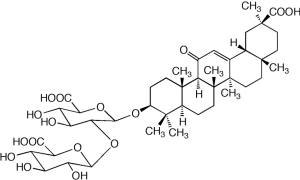
The molecular formula of GL was determined as C42H62O16 and molecular weight of GL was analyzed as 822.93, and the chemical structure of GL is presented in Figure 1. LPS (Escherichia coli serotype 0111: B4) was purchased from Sigma-Aldrich (St. Louis, MO, USA). TNF-α, IL-6, IL-1α, and enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN, USA). GL was supplied from LitePharmTech (Seoul, South Korea).
Animals
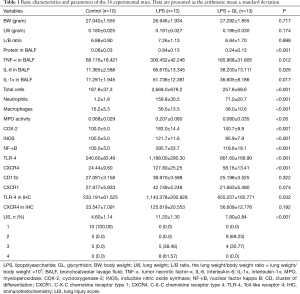
We used 6-week-old, specific pathogen-free male BALB/c mice (Japan SLC Inc., Shizuoka, Japan). The mice were acclimatized for 7 days to the KonKuk Laboratory Animal Research Center. They were conditioned at 21–23 °C with a constant humidity of 40–60% and a cycle of 12-hour light and 12-hour dark. The mice were maintained on food and tritiated water ad libitum. The mice were divided into 3 groups: those which received normal saline intravenously via a tail vein (control group, n=10), those which received LPS (50 mg/kg) intraperitoneally for the induction of ALI (LPS group, n=10), and those which received LPS for the induction of ALI and also received GL (50 mg/kg) twice via a tail vein (LPS + GL group, n=13). Mice were sacrificed on 24 hours after injection, and bronchoalveolar lavage fluid (BALF) was collected for the estimation of protein content, inflammatory cell counts, proinflammatory cytokines, myeloperoxidase (MPO) activity, and expressions of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and nuclear factor kappa B (NF-κB). Then, the lungs were excised for molecular target, histopathological and immunohistochemical examinations. The degree of lung injury and the therapeutic effect of GL were assessed 24 hours after LPS administration. All experimental animals were treated in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Konkuk University, Seoul, South Korea (KU 16048). The results are reported according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (6). Basic characteristics and parameters of the 36 experimental mice are summarized in Table 1, and data are presented as the arithmetic mean ± standard deviation.
Full table
Lung weight/body weight ratio
The lung weight/body weight ratio was calculated to measure water content in the lung tissue using the following formula: the lung weight/body weight ratio = lung weight/body weight ×103. Each mouse was anesthetized and the body weight was measured. After each mouse was euthanized, the lung was removed following ligation and cutting of the pulmonary artery and vein, and the lung weight was measured.
Examination of BALF
Each mouse was anesthetized by intraperitoneally administering a mixture of Zoletil® (tiletamine/zolazepam; Virbac Laboratories, Carros, France) and Rompun® (xylazine hydrochloride; Bayer Korea, Seoul, South Korea) at a 4:1 ratio (2 mL/kg). Then, a polyethylene tube was intubated. After 0.5 mL of Ca2+/Mg2+-free phosphate buffered saline was instilled and sucked out 3 times, BALF was collected. The BALF was centrifuged at 3,000 ×g at 4 °C for 10 minutes, and the supernatant was extracted.
Protein content
Protein content was measured using the Braford method. After 10 mL of BALF and 200 mL of Coomassie brilliant blue G-250 were placed and reacted on a 96-well microtiter plate for 5 minutes, absorbance was measured using a spectrophotometer at a wave of 595 nm relative to bovine serum albumin.
Inflammatory cell counts
After 10 mL of BALF and 10 mL of trypan blue were mixed, the total number of inflammatory cells (×104/mL BALF) was measured using a hemocytometer (Fischer Scientific, Pittsburgh, PA, USA). BALF cells were extracted using a cytospin and were smeared on a slide and stained for 10 minutes with Gimsa (Santa Cruz Biotechnology, Santa Cruz, PA, USA). The numbers of neutrophils and macrophages were calculated under a light microscope (×104/mL BALF).
Proinflammatory cytokines
The concentrations of TNF-α, IL-1α, and IL-6 were measured using sandwich ELISA kits (R&D systems, Minneapolis, MN, USA). A 96-well microtiter plate was coated with monoclonal antibodies specific for the aforementioned cytokines and was incubated with 300 mL of blocking buffer at room temperature for 1 hour. Then, 10 mL of BALF was transferred to the 96-well microtiter plate and was incubated with enzyme-linked polyclonal specific antibodies. Absorbance was measured within 30 minutes after addition of amplifier solution.
Measurement of MPO activity
BALF samples were sonicated with a sonicator for 10 seconds, and it was freezed and thawed several times. The BALF samples were centrifuged at 1,500 ×g at 4 °C for 10 minutes, and the supernatants were separated. Then, 50 mL of the supernatant were reacted with 0.169 mg/mL o-dianisidine hydrochloride and 0.0005% H2O2 on a 96-well microtiter plate. Absorbance was measured at 460 nm. MPO activity was measured 3 times in each BALF sample.
Western immunoblotting analysis
The expressions of COX-2, iNOS, and NF-κB in BALF were measured by using Western blotting analysis. The supernatant was diluted with sodium dodecyl sulfate (SDS) and was boiled at 100 °C for 10 minutes. After 20 mg of the protein sample was treated with 8% SDS-polyacrylamide gel electrophoresis, the protein was transcribed to polyvinylidene fluoride (PVDF) membrane (Immobilon-P; Millipore, Billerica, MA, USA). After nonspecific protein was blocked off the PVDF membrane for 60 minutes using blocking serum (5% nonfat milk in tris-buffered saline and Polysorbate 20), the PVDF membrane was reacted with primary antibody (rabbit antimurine COX-2, iNOS, and NF-κB polyclonal antibodies) at dilutions of 1:2,000 and 1:500 at 4 °C. Then, the membrane was reacted with secondary antibody conjugated with horseradish peroxidase at room temperature for 1 hour. It was treated with a color coupler (Western Lightening Chemiluminescence Reagent, Perkin-Elmer, Boston, MA, USA) and was developed on films. After that, the membrane was rinsed with Tris-buffered saline and Tween 20 and was washed with stripping butter (62.5 mM Tris-HCL pH 6.8, 2% SDS, 100 mM 2-mercaptoethanol) at 50 °C for 30 minutes. The membrane treated with stripping buffer underwent Western immunoblotting analysis using 1:2,000 goat anti-α-actin (Santa Cruz Biotechnology, Santa Cruz, PA, USA). The protein band was treated with enhanced chemiluminiscence, identified using LAS-4000 miniprogram, and assayed using Image J software ver. 1.41 (NIH, Bethesda, MD, USA).
Examination of the lung tissue
Histopathological examination using microscopy
After anesthesia, the mice were treated with perfusion and fixation using 1% paraformaldehyde solution (Biosesang, Seongnam, South Korea) and the lungs were extirpated. The extirpated lungs were embedded in paraffin blocks. The paraffin block was cut into 4-mm sections and stained with hematoxylin and eosin. The lung tissue was histopathologically examined under a light microscope (Eclipse Ni-E; Nikon, Tokyo, Japan) and was analyzed using Image J software ver. 1.41 (NIH).
Measurement of immunocytes migrating to the lung tissue
The lung tissue was cut into 1-mm3 cubic sections. The tissue was dissolved with collagenases for 30 minutes and was isolated into single cells using 100 mL of cell strainer. The immunocyte layer was separated after Ficoll density-gradient centrifugation at 2,400 ×g for 20 minutes. The isolated immunocytes were stained with CD11b-APCcy7, CXCR4-PerCP, and CXCR1-PE at room temperature for 30 minutes, and they were fixed with 4% paraformaldehyde for 10 minutes. The samples were analyzed using a high-speed flow cytometer (FACSAria; Automated High-speed Flow System, BD Biosciences, San Jose, CA, USA).
Immunohistochemistry
Immunohistochemical staining was conducted using the avidin-biotin-peroxidase (ABP) reagent (Vector Laboratories Inc., Burlingame, CA, USA). After the 4-mm-thick sections were deparaffinated with xylene, they were rehydrated and intrinsic H2O2 was blocked with 5% H2O2. The specimens were reacted with the primary antibodies anti-Toll-like receptor 4 (TLR-4) rabbit polyclonal antibody (Abcam, Cambridge, MA, USA), anti-CXCR4 rabbit monoclonal antibody (Abcam), and the secondary antibody biotinylated antibody (Vector Laboratories Inc.). Then, the samples were reacted with the ABP reagent (Vector Laboratories Inc.) and were treated with 3,30-diaminobenzidine reagent (Vector Laboratories Inc.). The samples were contrast-stained with hematoxylin (Vector Laboratories Inc.), examined using a light microscope (Eclipse Ni-E; Nikon), and analyzed using ImageJ software ver. 1.41 (NIH).
Statistical analysis
All data are presented as mean ± standard deviation. All statistical analyses were performed using SPPS for Windows Release 21.0 (IBM SPSS Inc. Armonk, NY, USA). Statistical comparisons were made using one-way analysis of variance with Tukey’s post hoc test. A P value of <0.05 was considered statistically significant.
Results
Effect of GL on the lung weight/body weight ratio
The lung weight/body weight ratio was measured to evaluate changes in capillary permeability and subsequent occurrence of pulmonary edema. The lung weight/body weight ratio was slightly increased in the LPS group compared to the control group (control vs. LPS vs. LPS + GL, 6.88±0.90 vs. 7.26±1.13 vs. 6.84±1.70, P=0.898), but it does not show any statistical significance (P=0.898) (Figure 2A).
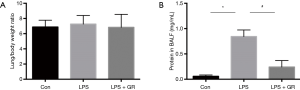
Effect of GL on protein content in BALF
The protein content in BALF was measured to evaluate protein leak due to alveolar capillary injury. The protein level was significantly more increased in the LPS group than in the control group, while it was significantly more decreased in the LPS + GL group than in the control group (Figure 2B).
Effect of GL on the numbers of inflammatory cells in BALF
The numbers of total cells, neutrophils, and macrophages were significantly more increased in the LPS group than in the control group, while they were significantly more decreased in the LPS + GL group than in the control group. The number of total cells in the LPS + GL group was decreased to that in the control group; however, the numbers of neutrophils and macrophages were larger in the LPS + GL group than in the control group (Figure 3A,B,C).
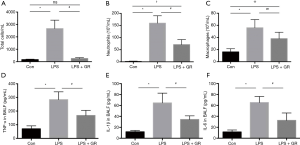
Effect of GL on the levels of proinflammatory cytokines in BALF
The proinflammatory cytokines TNF-α, IL-1α, and IL-6 were significantly more increased but more decreased in the LPS group than in the control group (Figure 3D,E,F).
Effect of GL on MPO activity in BALF
MPO activity was measured to evaluate the infiltration degree of neutrophils and the severity of lung injury. The MPO activity was significantly more increased in the LPS group than in the control group, while it was significantly more decreased in the LPS + GL group than in the LPS group. However, there was no significant difference in the MPO activity between the LPS + GL and control groups (Figure 4A).
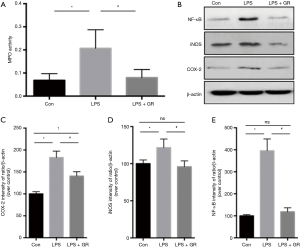
Effect of GL on expressions of COX-2, iNOS, and NF-κB in BALF
The COX-2 expression was significantly higher in the LPS group than in the control group. In the LPS + GL group, the COX-2 expression was lower than in the LPS group, but higher than in the control group. The expressions of iNOS and NF-κB were higher in the LPS group than in the control group, while they were lower in the LPS + GL group than in the LPS group (Figure 4B,C,D,E).
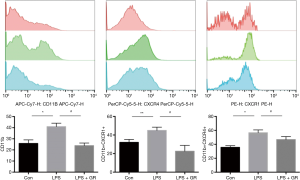
Effect of GL on expressions of CD11b−/CXCR4−/CXCR1-positive cells in BALF
The expressions of CD11b−/CXCR4−/CXCR1-positive cells were significantly higher in the LPS group than in the control group, while they were significantly lower in the LPS + GL group than in the LPS group (Figure 5).
Effect of GL on expressions of TLR-4 and CXCR4 in the lung tissue obtained from a mouse ALI model
The TLR-4 expression was significantly more increased in the LPS group than in the control group, while it was significantly more decreased in the LPS + GL group than in the LPS group (Figures 6,7A,B). The degree of inflammation was quantified by using the lung injury score. Alveolar capillary congestion, hemorrhage, inflammatory cell infiltration into the alveolar space and interstitium, and alveolar wall thickening/hyaline membrane formation were rated on a 5-point scale: 0, absent or minimal; 1, mild; 2, moderate; 3, severe; and 4, maximal. The lung injury score was significantly higher in the LPS group than in the control group, while it was significantly lower in the LPS + GL group than in the LPS group (Figure 7C).
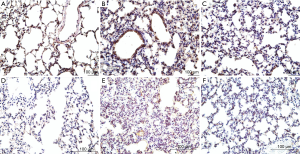

Effect of GL on histopathological findings in the lung tissue obtained from a mouse ALI model
There were severe inflammatory reactions, including inflammatory cell infiltration, alveolar congestion, alveolar wall thickening, and hemorrhage, in the LPS group; however, the reactions were milder in the LPS + GL than in the LPS group (Figure 8A,B,C).

Discussion
Bilateral acute hypoxemic respiratory failure, which had been known as shock lung or adult respiratory distress syndrome, was termed ARDS by Ashbaugh et al. (7). They also stated, in a study of 12 adult patients, that the characteristic features of ARDS were acute-onset dyspnea, tachypnea, cyanosis refractory to oxygenation therapy, loss of lung compliance, and diffuse alveolar infiltration on chest X-ray. The clinical characteristics of ARDS include hypoxemia and bilateral pulmonary infiltration, and its pathological features include alveolar edema, acute alveolar wall inflammation, hyaline membrane formation, and diffuse lung injury. The 4-point lung injury score system and diagnostic criteria for ARDS proposed by the American-European Consensus Conference have been replaced by the Berlin Definition in 1988 (8). The Berlin Definition proposed that ARDS can be diagnosed when: (I) respiratory symptoms develop within 7 days; (II) chest X-rays or chest computed tomographic scans shows bilateral pulmonary edema accompanied by pulmonary emphysema; (III) cardiac failure or fluid overload is ruled out as its cause; (IV) there is moderate to severe hypoxemia. The Berlin Definition proposed 3 mutually exclusive categories of ARDS based on the degree of hypoxemia: mild (200 mmHg < PaO2/FiO2 ≤300 mmHg), moderate (100 mmHg < PaO2/FiO2 ≤200 mmHg), and severe (PaO2/FiO2 ≤100 mmHg). These categories are useful to predict patient prognosis. In the United States, approximately 190,000 patients are diagnosed with ARDS each year; however, they have been treated only with mechanical ventilation, pone positioning, steroid therapy, administration of nitric oxide, and extracorporeal membrane oxygenation, with high mortality rates. Recent studies on ARDS have focused on the exploration of sera or biomarkers involved in the occurrence and progression of ALI, and many investigators have attempted to attenuate ALI and inhibit the progression to ARDS (9). Proinflammatory cytokines have been recognized as the most useful biomarkers for predicting complications and clinical outcomes of inflammatory diseases. Terpstra et al. (10) performed a meta-analysis of 54 studies involving 3,753 ARDS patients and demonstrated that IL-4, IL-6, angioprotein-2, Krebs von den Lungen-6, and protein C were closely related to patient mortality. In animals, ARDS has been induced by using various methods, including mechanical ventilation, ischemia/reperfusion, irradiation, bleomycin administration, and LPS administration. ALI/ARDS can be caused by more than 60 etiologies, of which sepsis is the most common etiology. In the present study, LPS was used to induce ARDS in mice because a mouse ARDS model has been regarded as a suitable animal model to conduct studies on ARDS. LPS stimulates macrophages to secret proinflammatory cytokines and adhesion molecules, provokes the inflammatory cascade by increasing the migration and infiltration of immunocytes related to inflammation through chemokines, and leads to ALI.
GL is a natural extract from the roots of Glycyrrhiza glabra, Glycyrrhiza uralensis, and Glycyrrhiza inflate, and it consists of glycyrrhetinic acid and glucuronic acid. This compound has a molecular weight of 822.94 and has been used as a natural sweetener as it is 50 times sweeter than sugar. GL has a low absorption rate in the gastrointestinal tract; however, it is hydrolyzed into glycyrrhetinic acid to be absorbed, which is metabolized in the liver and excreted in bile in large amounts and in urine in small amounts. Although the absorption, distribution, metabolism, and excretion of GL are similar between intravenous injection and intraperitoneal administration, its in vivo use is limited because it is absorbed in small amounts via intraoral administration (11). In the United States, GL is generally recognized as safe and is used as an additive to liquors, drinks, candies, and cigarettes, but the Food and Drug Administration proposes the maximum concentration of GL for individual foods. The European Union’s Scientific Committee recommends a maximum concentration of 100 mg/day in food. There have been numerous studies on pharmacological effects of GL and its derivatives. Sheela et al. (12) identified that GL extract inhibited the in vivo and in vitro proliferation of Ehrlich ascites tumors cells as well as decreased vascular endothelial growth factor production and neovascularization. They suggested that GL extract may be a potential supplemental source for cancer therapy, and glycyrrhizic acid (GA) has a chemoprotective effect in 1,2-dimethyhydrazine-induced precancerous lesions by suppressing their development. GL has been shown to exert antidiabetic, antidyslipidemic, and antiarteriosclerotic effects. Takii et al. (13) evaluated the effects of GL treatment using genetically noninsulin-dependent diabetes mice by dividing the mice into 3 groups: the control group, the 0.27% GL diet group, and the 0.41% GL diet group. Cheng et al. (14) investigated GA effects on glucose and lipid metabolism in 24 male Sprague Dawley rats, and advocated that high-fat high-sucrose diet-induced hyperglycemia, insulin resistance, and dyslipidemia are attenuated by supplementation with GA. In an experimental study with high-fat diet-induced obese rats, GL attenuates dyslipidemia by inducing lipoprotein lipase expression and lipoprotein production (15). GL has been reported to have protective effects in the liver. In a study with a rat model, GL has a therapeutic effect on retrosine-induced hepatotoxicity by reducing the serum levels of glutamate oxaloacetate transaminase and glutamate pyruvate transaminase (16). There have been numerous studies on antiviral effects of GL. GL can be used as a therapeutic agent in influenza patients and inhibits apoptosis and fibrosis in carborrtetrachloride-induced rat liver injury (17). Gao et al. (18) identified that GL attenuates lung fibrosis in a bleomycin-induced lung fibrosis rat model and proposed that GL can be useful for preventing fibrosis in ALI. Many studies have explored anti-inflammatory effects of GL and its mechanism of action in various inflammatory diseases, such as colitis, pancreatitis, myocarditis, and chronic dermatitis. Previous studies have reported several side effects of GL, including hypertension and generalized edema. GL may induce excessive calcium excretion, sodium/water retention, weight gain, alkalosis, inhibition of the rennin-angiotensin-aldosterone system, hypertension, and muscle paralysis, which result from activation of the mineralocorticoid receptors of distal renal tubules. Since cortisol, a glucocorticoid, is similar in structure to aldosterone, a mineralocorticoid, it can bind to mineralocorticoid receptors. Blood cortisol concentration is 100–1,000 times higher than blood aldosterone concentration, so that 11α-hydroxysteroid dehydrogenase-2 (11α-OHSD2) converts cortisol into inactive cortisone to prevent hyperstimulation of mineralocorticoid receptors. GL induces generalized symptoms by suppressing 11α-OHSD2 activity in the renal tubules. In the present study, we first measured the lung weight/body weight ratio, protein content, COX-2 expression, and iNOS expression as well as performed histopathological examination to assess the effects of GL on pulmonary edema and lung injury. Next, we measured proinflammatory cytokine concentration, TLR-4 expression, and NF-κB expression to evaluate the effects of GL on the initial phase of inflammation and its mechanism of action. Finally, we measured the number of inflammatory cells, MPO activity, and CD11B−/CXCR1-positive cell expression to determine the inhibitory effects of GL on neutrophils. In the present study, we found that GL has no significant effect on the lung weight/body weight ratio, but ameliorate pulmonary edema by a significant reduction in protein content. We also identified that GL had a therapeutic effect on pulmonary edema and ALI by histopathological examination. Prostanoids are synthesized from arachidonic acid by cyclooxygenase activity, and induce pain and fever in the initial phase of inflammation. COX-2 is absent in most of the normal tissues, but it is induced by proflammatory cytokines, LPS, and carcinogens. The role of COX-2 in pancreatitis and its associated lung injury using both genetically altered and pharmacologically inhibited mice, and suggested that the severity of pancreatitis and its associated lung injury were more ameliorated in the inhibited mice compared to the noninhibited strains of COX-2-sufficient mice, and that COX-2 may play an important role in pancreatitis and its associated lung injury (19). Inhibition of COX-2 prior to endotoxin and oleic acid administration significantly reduced the plasma and lung tissue concentrations of prostacyclin compared to those in the group given only endotoxin + oleic acid. The loss of pulmonary perfusion, as assessed by positron emission tomography, was induced by a COX-2-mediated increase in prostacyclin production in the lung tissue. Blockage of COX-2 gene expression and COX-2 inhibition can suppress lung injury and inflammation. Isofraxidin has a protective effect against LPS-induced ALI in mice and that the protective effect may result from the inhibition of COX-2 protein expression in the lung, which regulates prostaglandin E2 production (20). In an analysis of PubMed databases by Huang et al., they proved that GL can be a therapeutic agent for rheumatoid arthritis via suppressing COX-2/thromboxane A2 (21). In the present study, we identified that COX-2 gene expression increased by LPS administration can be attenuated by GL administration, and that GL significantly ameliorates LPS-induced iNOS expression. Proinflammatory cytokines play an important role in the initial phase of inflammation, and increased cytokine levels represent severe inflammation. The levels of TNF-α and IL-6 are increased in BALF from ALI patients and that increases in proinflammatory cytokines are closely related to clinical outcomes of ALI patients (22). The benefit of GL in a mouse model of carbon tetrachloride (CCl4)-induced liver injury. The mice were treated intraperitoneally with CCl4 (0.5 mg/kg) and received GL at 50, 100, 200, and 400 mg/kg 24 and 0.5 hours before CCl4 administration, and 4 hours after CCL4 administration. GL reduced the increased level of TNF-α as well as the expressions of iNOS and COX-2, and may protect liver injury via inducing heme oxygenase-1 and down-regulating proinflammatory cytokines (23). The recent study, 2016, by Zhao et al. (24), reported the mechanisms and effects of GL against sepsis-induced ALI in rats. They found that GL alleviated sepsis-induced ALI by various pathological changes, including a significant decrease in the lung wet/dry weight ratio and total protein content in BALF and a meaningful increase in the survival rate of rats. They used relatively high-weighted rats (200±20 gram), pathogen-free male Sprague-Dawley rats, but we used relatively small sized mice (27.0±2.0 gram), 6-week-old, specific pathogen-free male BALB/c mice. For the sepsis-induced ALI experimental model, they induced an iatrogenic peritonitis by a method of an injection with sodium pentobarbital (50 mg/kg), a laparotomy, twice cecum puncture at different sites and feces extrusion. However, we made an intraperitoneal injection of LPS (50 mg/kg) for the induction of LPS-induced ALI. In the year 2019, Kong et al. (25) demonstrated that GL has an enrichment of function as a suppressor in TLR signaling pathway to reduce LPS-induced ALI by inhibiting TLR-2. For the LPS-induced ALI model, they applied a dissolved LPS in saline at a concentration of 500 µg/kg and injected an LPS at a volume of 1 mL/kg/body weight through intratracheal administration. However, we directly performed an intraperitoneal injection of LPS (50 mg/kg) for the induction of LPS-induced ALI. Subsequently, GL 1 mg was instilled in the airways of mice in the LPS + GL group just before intratracheal administration of LPS, and mice in the LPS plus saline group received 2 mL of saline immediately. However, compared to Kong and his colleagues’ regimens, we used the same concentration of LPS (500 µg/kg), but a high concentration of GL, 50 mg/kg, was administrated twice via a tail vein. Zhang et al. (26) determined the therapeutic effect of GL on Coxsackie virus B3-induced myocarditis and its possible mechanisms involved. GL improved weight loss, decreased the levels of cardiac enzymes, ameliorated myocardial inflammation, and prolonged survival rate. They showed that these effects were not attributed to viral clearance but to reduced expressions of proinflammatory cytokines including TNF-α, IL-1β, and IL-6. They also concluded that GL can be a new therapeutic agent for viral myocarditis. Recent studies have focused on NF-κB activation for secretion of proinflammatory cytokines via regulating TLR-4 signaling (27). The human TLR was first described in 1944. Since then, TLR family members have been reported in the literature. Human TRLs recognize pathogens in the initial phase of inflammation and play a crucial role in activating the innate immune system. Human TLRs function as pattern-recognition receptors in innate immunity, which are expressed on sentinel cells, such as macrophages and dendritic cells. Among the human LTRs, TLR-4 recognizes the external wall of Gram-negative bacteria. LPS binds to the extracellular proteins LPS-binding protein, glycosylphosphatidylinositol-anchored protein (CD14), and myeloid differentiation protein 2 to activate TLR-4. This TLR-4 activates NF-κB via regulating the myeloid differentiation primary response gene 88 (MyD88) and interferon regulatory factor 3 (IRF3) via regulating toll/IL-1 receptor-domain-containing adapter-inducing interferon-α-dependent signal pathways. NF-κB is a transcription factor involved in the expressions of proinflammatory cytokines, such as high-mobility group box 1 (HMGB1), TNF-α, and IL-1β. IRF3 is a transcription factor involved in the expression of interferon. Therefore, TLR-4 activated by LPS induces the expressions of NF-κB and IRF3 which secret proinflammatory cytokines. Based on these results, it is conceivable that TLR-4 blocking can be a potential strategy for treating inflammatory disease. He et al. (27) investigated the effect of TLR-4 monoclonal antibody (mAb) on LPS-induced ALI in mice. A total of 45 male BALB/c mice were divided into 3 groups: the control, LPS-induced sepsis, and pretreatment groups. The mice in the pretreatment group were intraperitoneally treated with TLR-4 mAb 1 hour before intraperitoneal LPS administration. TLR-4 expression, secretion of proinflammatory cytokines, and the degree of pulmonary edema/lung injury were reduced in the pretreatment group, and they concluded that TLR-4 plays a key role in LPS-induced ALI. Fu et al. (28) conducted in vivo and in vitro experiments using an LPS-induced endotoxemia mouse model and RAW264.7 cells, respectively. In vivo, GL improved survival during lethal endotoxemia; in vitro GL inhibited the expressions of TNF-α, IL-6, IL-1β, and regulated on activation, normal T cell expressed and secreted factor, and suppressed LPS-induced LF-KB and IRF3 activation. They concluded that GL exerts an anti-inflammatory effect by inhibiting translocation of TLR-4 to lipid rafts. In the present study, it was found that GL suppressed the expressions of TLR-4 and NF-κB. Immunocytes, especially neutrophils, play a crucial role in provoking inflammation, and their mechanism of action for ALI contributes to better understanding of ARDS. They also found that the dosage of 20 mg/kg LPS could be lethal to induce endotoxemia in mice. In the present study, we identified that GL significantly reduced the numbers of total cells, neutrophils, and macrophages. MPO has a potent antibacterial activity and is abundantly contained in eosinophil granules. Based on these results, increased MPO activity suggests the migration and infiltration of neutrophils, and MPO activity can be used as an indicator of the severity of inflammation and ling injury. Haegens et al. (29) observed that neutrophil infiltration and proinflammatory cell production were decreased in MPO (−/−) mice treated with LPS, and concluded that MPO promotes the development of lung neutrophilia and influences production of chemokines and cytokines.
Complement receptor type 3 (CR3, CD11b/CD18, and Mac-1, Mo1) is a member of the β2-integrin family that also includes lymphocyte function antigen-1 (EFA-1; CD11a/CD18) and protein 150, 95 (p150, 95; CD11c/CD18), each of which consists of subunits CD11b and CD18. CR3 is expressed on the surfaces of CD11b-rich immunocytes, such as monocytes, granulocytes, macrophages, and natural killer cells, and it also plays an important role in the innate immune system. CR3 has been known to induce inflammation via regulating the migration and infiltration of neutrophils and to be involved in various immunological reactions including phagocytosis, cell-mediated cytotoxicity, chemotaxis, and cell activation. Although CD11b is directly involved in cell adhesion and perfusion, it can mediate cell migration only in the presence of CD18. Moreland et al. (30) evaluated the role of CD11b in LPS-induced pulmonary disease. C57BL/6J mice receiving anti-CD11b antibody showed a significant reduction in pulmonary neutrophil recruitment compared to control mice (18,300 vs. 143,000 cells/mL; neutrophils 16.7% vs. 77%). They concluded that CD11b is an important mediator of LPS-induced pulmonary inflammation. Zeni et al. (31) reported that among rats challenged with intrabronchial E. coil, there were a lesser degree of lung injury and a smaller number of neutrophils in those treated with anti-CD11b antibody, and concluded that CD11b is related to the migration and infiltration of neutrophils. It has been shown that when C-X-C chemokine receptor type 4 (CXCR4) specific for stromal-derived factor-1 (SDF-1 or CXCL12) and IL-8 receptor-α (CXCR1) specific for IL-8 are activated, the proliferation, migration, and infiltration of inflammatory cells are increased via the intracellular signal system. Drummond et al. (32) examined whether CXCR4 antagonists could attenuate hyperoxia-induced lung injury in neonatal rats. Newborn mice receiving the CXCR4 antagonist AMD3100 had reduced lung inflammation and showed improved alveolarization and increased angiogenesis. They concluded that CXCR4 antagonists can be a novel strategy for alleviating lung injury in preterm infants with bronchopulmonary dysplasia. Schneberger et al. (33) documented that CXCR1 antagonists attenuate pulmonary inflammation. Schiraldi et al. (34) indicated that HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and by signaling via CXCR4, which is suppressed by AMD3100 and GL. The increases in CD11b-positive cells and CXCR4−/CXCR1-expressing cells suggest that neutrophil and other immunocytes may migrate and infiltrate into lung tissue by severe inflammatory reactions. In the present study, it was found that GL blocked the influx of CD11b-positive cells and CXCR4−/CXCR1-expressing cells into lung tissue.
It is relatively well reported that the glycyrrhetic acid and GL inhibits the 11-beta-hydroxysteroid dehydrogenase enzyme type 2, which catalyze the conversion of inert 11-keto-products (cortisone) to active cortisol, results a cortisol-induced mineralocorticoid effect and has a potent relationship on elevation of plasma sodium level and reduction of plasma potassium level. The aldosterone-like action by the GL is considered as the various spectrum of adverse and pharmacological effects, including anti-inflammatory, anti-tumor, hepato-protective, anti-viral and therapeutic effect on ALI. GL and glycyrrhetic acid, the active metabolite in licorice, are approved as a food supplement under the US Food and Drug Administration (FDA). The FDA suggests that GL oral intake should not exceed 100 mg/day, because overdose of GL can induce the mineralocorticoid-like effect, and serious life-threatening complications can occur with excess GL use. Inhibition of 11-beta-hydroxisteroid dehydrogenase by GL overdose leads to a consequent reabsorption of sodium and excretion of potassium, which is defined as pseudo-hyperaldosteronism. Most common clinical signs on GL overdose are mainly hypertension and hypokalemia (35). For the clinical application of GL, it would be important to define the therapeutic dosage and the appropriate administration route of GL. On the basis published papers, there are pretty different opinions on the proper dosage of GL from minimal 1.0 mg/kg to maximal 400 mg/kg. More detailed information and systematic review of the literature on GL dosage were summarized in Table 2.
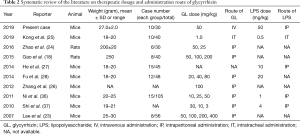
Full table
Conclusions
In this study, it was observed that GL had an anti-inflammatory effect on damaged lung tissue in an ALI mouse model. GL attenuated inflammatory cell infiltration, pulmonary edema, and histopathological changes. It is conceivable that GL reduces cytokine secretion via blocking NF-κB activation by the intracellular signal pathway and inhibits the migration and infiltration of neutrophils via decreasing CXCR4/CXCR1 expression. The results of the present study suggest that GL can be used as a novel therapeutic strategy for pulmonary inflammation.
Acknowledgements
This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2013.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All experimental animals were treated in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Konkuk University, Seoul, South Korea (KU 16048).
References
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Kim DH, Kim ME, Lee JS. Inhibitory effects of extract from G. lanceolata on LPS-induced production of nitric oxide and IL-1â via down-regulation of MAPK in macrophages. Appl Biochem Biotechnol 2015;175:657-65. [Crossref] [PubMed]
- Hashimoto S, Okayama Y, Shime N, et al. Neutrophil elastase activity in acute lung injury and respiratory distress syndrome. Respirology 2008;13:581-4. [Crossref] [PubMed]
- Abdelmageed ME, El-Awady MS, Suddek GM. Apocynin ameliorates endotoxin-induced acute lung injury in rats. Int Immunopharmacol 2016;30:163-70. [Crossref] [PubMed]
- Qamar W, Khan R, Khan AQ, et al. Alleviation of lung injury by glycyrrhizic acid in benzo(a)pyrene exposed rats: Probable role of soluble epoxide hydrolase and thioredoxin reductase. Toxicology 2012;291:25-31. [Crossref] [PubMed]
- Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010;8:e1000412. [Crossref] [PubMed]
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526-33. [PubMed]
- Riviello ED, Kiviri W, Twagirumugabe T, et al. Hospital Incidence and Outcomes of the Acute Respiratory Distress Syndrome Using the Kigali Modification of the Berlin Definition. Am J Respir Crit Care Med 2016;193:52-9. [Crossref] [PubMed]
- Terpstra ML, Aman J, van Nieuw Amerongen GP, et al. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med 2014;42:691-700. [Crossref] [PubMed]
- Yamamura Y, Santa T, Kotaki H, et al. Administration-route dependency of absorption of glycyrrhizin in rats: intraperitoneal administration dramatically enhanced bioavailability. Biol Pharm Bull 1995;18:337-41. [Crossref] [PubMed]
- Sheela ML, Ramakrishna MK, Salimath BP. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int Immunopharmacol 2006;6:494-8. [Crossref] [PubMed]
- Takii H, Kometani T, Nishimura T, et al. Antidiabetic effect of glycyrrhizin in genetically diabetic KK-Ay mice. Biol Pharm Bull 2001;24:484-7. [Crossref] [PubMed]
- Cheng HS, Yaw HP, Ton SH, et al. Glycyrrhizic acid prevents high calorie diet-induced metabolic aberrations despite the suppression of peroxisome proliferator-activated receptor y expression. Nutrition 2016;32:995-1001. [Crossref] [PubMed]
- Eu CH, Lim WY, Ton SH, et al. Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis 2010;9:81. [Crossref] [PubMed]
- Lin G, Nnane IP, Cheng TY. The effects of pretreatment with glycyrrhizin and glycyrrhetinic acid on the retrorsine-induced hepatotoxicity inrats. Toxicon 1999;37:1259-70. [Crossref] [PubMed]
- Wolkerstorfer A, Kurz H, Bachhofner N, et al. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Res 2009;83:171-8. [Crossref] [PubMed]
- Gao L, Tang H, He H, et al. Glycyrrhizic acid alleviates bleomycin-induced pulmonary fibrosis in rats. Front Pharmacol 2015;6:215. [Crossref] [PubMed]
- Song AM, Bhagat L, Singh VP, et al. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 2002;283:G1166-74. [Crossref] [PubMed]
- Niu X, Wang Y, Li W, et al. Protective effects of Isofraxidin against lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol 2015;24:432-9. [Crossref] [PubMed]
- Huang QC, Wang MJ, Chen XM, et al. Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis? Oncotarget 2016;7:1193-202. [PubMed]
- Minamino T, Komuro I. Regeneration of the endothelium as a novel therapeutic strategy for acute lung injury. J Clin Invest 2006;116:2316-9. [Crossref] [PubMed]
- Lee CH, Park SW, Kim YS, et al. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol Pharm Bull 2007;30:1898-904. [Crossref] [PubMed]
- Zhao H, Zhao M, Wang Y, et al. Glycyrrhizic Acid Prevents Sepsis-Induced Acute Lung Injury and Mortality in Rats. J Histochem Cytochem 2016;64:125-37. [Crossref] [PubMed]
- Kong D, Wang Z, Tian J, et al. Glycyrrhizin inactivates toll-like receptor (TLR) signaling pathway to reduce lipopolysaccharide-induced acute lung injury by inhibiting TLR2. J Cell Physiol 2019;234:4597-607. [Crossref] [PubMed]
- Zhang H, Song Y, Zhang Z. Glycyrrhizin administration ameliorates coxsackie virus B3-induced myocarditis in mice. Am J Med Sci 2012;344:206-10. [Crossref] [PubMed]
- He Z, Chen X, Wang S, Zou Z. Toll-like receptor 4 monoclonal antibody attenuates lipopolysaccharide-induced acute lung injury in mice. Exp Ther Med 2014;8:871-6. [Crossref] [PubMed]
- Fu Y, Zhou E, Wei Z, et al. Glycyrrhizin inhibits lipopolysaccharide-induced inflammatory response by reducing TLR4 recruitment into lipid rafts in RAW264.7 cells. Biochim Biophys Acta 2014;1840:1755-64. [Crossref] [PubMed]
- Haegens A, Heeringa P, van Suylen RJ, et al. Myeloperoxidase deficiency attenuates lipopolysaccharide-induced acute lung inflammation and subsequent cytokine and chemokine production. J Immunol 2009;182:7990-6. [Crossref] [PubMed]
- Moreland JG, Fuhrman RM, Pruessner JA, et al. CD11b and intercellular adhesion molecule-1 are involved in pulmonary neutrophil recruitment in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol 2002;27:474-80. [Crossref] [PubMed]
- Zeni F, Parent C, Correa R, et al. ICAM-1 and CD11b inhibition worsen outcome in rats with E. coli pneumonia. J Appl Physiol 1999;87:299-307. [Crossref] [PubMed]
- Drummond S, Ramachandran S, Torres E, et al. CXCR4 blockade attenuates hyperoxia-induced lung injury in neonatal rats. Neonatology 2015;107:304-11. [Crossref] [PubMed]
- Schneberger D, Gordon JR, DeVasure JM, et al. CXCR1/CXCR2 antagonist CXCL8(3-74)K11R/G31P blocks lung inflammation in swine barn dust-instilled mice. Pulm Pharmacol Ther 2015;31:55-62. [Crossref] [PubMed]
- Schiraldi M, Raucci A, Muñoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012;209:551-63. [Crossref] [PubMed]
- Omar HR, Komarova I, El-Ghonemi M, et al. Licorice abuse: time to send a warning message. Ther Adv Endocrinol Metab 2012;3:125-38. [Crossref] [PubMed]
- Ni YF, Kuai JK, Lu ZF, et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J Surg Res 2011;165:e29-35. [Crossref] [PubMed]
- Shi JR, Mao LG, Jiang RA, et al. Monoammonium glycyrrhizinate inhibited the inflammation of LPS-induced acute lung injury in mice. Int Immunopharmacol 2010;10:1235-41. [Crossref] [PubMed]