
Cell therapy for acute respiratory distress syndrome patients: the START study
Every year, 200,000 new cases of acute respiratory distress syndrome (ARDS) occur in the United States alone, with an incidence of 10% among patients admitted to intensive care units (ICU) and a mortality rate of 30–40% (1). In recent decades, new therapeutic strategies have been developed; nevertheless, none has proven effective. Therefore, the management of ARDS is still based on supportive care strategies, such as lung-protective mechanical ventilation and conservative fluid administration. In several experimental models of ARDS, either intravenous or intratracheal administration of mesenchymal stromal cells (MSCs) obtained from various sources (bone marrow, adipose tissue, lung, cord blood, dental pulp, and placenta) resulted in beneficial effects (2). MSCs have been shown to reduce inflammation and alveolar oedema, augment tissue repair, enhance pathogen clearance, improve lung mechanics, and reduce disease associated-mortality (3) in experimental ARDS, prompting the design of clinical trials of this therapy.
In Lancet Respir Med (4), Wilson and colleagues reported the results of the phase-I STem cells for ARDS Treatment (START) study. Patients with moderate-to-severe ARDS received a single intravenous administration of low-dose [106 MSCs/kg predicted body weight (PBW)], intermediate-dose (5×106 MSCs/kg PBW), or high-dose MSCs (107 MSCs/kg PBW) (n=3/dose) (4). No evidence of infusion-related clinical instability, adverse events, or toxicity was observed at any of the doses tested. High-dose MSCs improved daily SOFA score compared to lower doses (4). Altogether, these results suggest that the beneficial effects of cell therapy in ARDS patients are associated with higher doses of MSCs.
Based on these promising results, the same group continued the START study into phase IIa (5). Matthay and colleagues reported a prospective, double-blind, randomized clinical trial evaluating the effects of a single systemic dose of allogeneic bone marrow-derived MSCs (107 cells/PBW) compared with placebo (2:1 ratio). They recruited mechanically ventilated patients with moderate-to-severe ARDS, a PaO2/FiO2 ratio <200 mmHg, a positive end-expiratory airway pressure (PEEP) of at least 8 cmH2O, and non-cardiogenic bilateral lung infiltrates on frontal chest radiograph. The primary outcome was safety, and the secondary outcomes included respiratory, systemic, and serum biomarker endpoints (5). Sixty patients were enrolled in this study. No adverse respiratory or hemodynamic events were observed during MSC administration or 6 hours after injection. However, there were no statistical differences between groups concerning mortality at day 28 [30% (MSC) vs. 15% (placebo)], mortality at day 60 [38% (MSC) vs. 25% (placebo)], number of ventilator-free days at day 28 [2 (MSC) vs. 17 (placebo)], or number of ICU-free days at day 28 [2 (MSC) vs. 14 (placebo)]. ARDS patients presented numerical differences in disease severity scores at baseline. In this line, the placebo group had better Acute Physiology and Chronic Health Evaluation III (APACHE III) scores (104±31 vs. 89±33), minute ventilation (11.1±3.2 vs. 9.6±2.4 L/min), and PEEP (12.4±3.7 vs. 10.8±2.6 cmH2O) than the MSC group (5). Mortality rate in the placebo group was lower than expected based on disease severity (1). On the other hand, ARDS patients treated with MSCs exhibited reduced systemic levels of angiopoietin-2. The authors reported an unexpected finding: despite adequate cell viability at the time of manufacturing, substantial variation in MSC viability was observed at the time of intravenous injection (36% to 85%). Finally, there was a strong indirect correlation between cell viability and levels of angiopoietin-2, as well as between viability and improvement in oxygenation index (5). This variability in viability may have played a role in the lack of efficacy, but MSC biology is still not fully understood. Different clonal subpopulations can be obtained from the same bone marrow aspirate. The immunomodulatory and regenerative properties of MSCs can be modulated by different tissue culture procedures, reagents, passage, confluence, and other factors (6). Cryopreservation can have several impacts on cell receptors and on the secretome (7); however, fresh MSCs can induce the same in vivo effects as frozen ones (8,9). Additionally, the role of cell death in the functional effects of MSCs has been discussed. MSCs undergo autophagy (10), apoptosis (11), and efferocytosis (12,13) after intravenous administration. It is believed that the host inflammatory microenvironment, leukocytes, and plasma components such as the complement system can induce cell death (10-13). Some authors believe that MSC death is related to the lack of persistence of their effects (14). Others, conversely, believe that MSC death is protective and even necessary to promote their immunoregulatory effects. In other inflammatory conditions, such as graft-versus-host disease, the host’s cytotoxic ability to induce MSC cell death correlates directly with the success of cell therapy (15). Thus, the real impact of cell viability at the time of administration has yet to be elucidated.
Other studies have tested MSC therapy in patients with ARDS, but were heterogeneous in terms of MSC source, number of administered cells, administration route, inclusion and exclusion criteria, and follow-up duration. Their main goal has been to evaluate the safety of MSC therapy, and secondarily, to analyse effects on respiratory, cardiovascular, and inflammatory parameters. These clinical studies had small sample sizes, thus decreasing their statistical power.
In China, Zheng et al. carried out a phase I, double-blind, placebo-controlled trial assessing the safety of intravenous administration of human adipose tissue-derived MSCs (NCT01902082) in patients with ARDS (16). Cell therapy (1×106 cells/kg) appeared to be safe. After MSC administration, a short-term improvement was observed in oxygenation, but ventilator-free days, ICU-free days, and length of hospital stay were unchanged (16). Limitations included the short duration of follow-up (28 days) and the lack of dose-response and time-response data for MSCs. In contrast to the findings observed by Zheng et al., researchers in Sweden tested systemic administration of allogeneic bone marrow-derived MSCs (2×106 cells/kg) in two patients with severe refractory ARDS who had failed to improve after all supportive therapies, i.e., in a compassionate use setting (17). Both patients recovered from multiple organ failure and presented reduced systemic and pulmonary markers of inflammation (17). Recently, in a press release, Athersys announced positive results of a randomised, placebo-controlled, phase I/II study conducted in the USA and UK which aimed to test the safety and possible efficacy of the adult stem-cell investigational product MultiStem® in patients with ARDS (NCT 02611609, registry number at clinicaltrial.gov). MSC therapy was safe and reduced mortality rate, ventilator-free days, and ICU-free days in the first month following diagnosis compared to placebo. Furthermore, analysis of initial biomarker data revealed lower levels of inflammatory cytokines following MultiStem® treatment, an expected mechanism of action in this patient population. Patients in the exploratory study were evaluated through 28 days for primary clinical assessment and will be further assessed through a 1-year follow-up period. Whether the different results found among groups reflect disparities generated by MSC source (bone-marrow vs. adipose-tissue) or any other discrepancy between protocols still needs to be determined.
Several other clinical trials are ongoing (Table 1). A group from Sweden is conducting a phase I, multicentre, non-randomised, controlled, open-label safety trial assessing the impact of bone marrow-derived MSCs in viral-induced ARDS under mechanical ventilation and extracorporeal membrane oxygenation (NCT02215811). Three independent groups are also running phase I trials in China to test therapy with multiple doses of menstrual blood-derived (NCT02095444) and umbilical cord-derived (NCT02444455) and (NCT03608592) MSCs in ARDS. A group in the UK is recruiting ARDS patients to evaluate the effects of human umbilical cord derived CD362 enriched MSCs. In USA, two groups will assess bone marrow-MSC therapy in ARDS patients [(NCT02804945) and (NCT03818854)]. Lastly, STELLAR, a phase I study conducted in Korea (NCT02112500), is exploring the impact of bone marrow-derived MSCs in patients with ARDS.
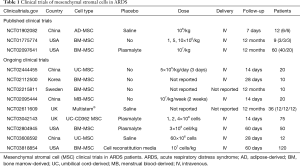
Full table
In short, clinical studies have reported that MSC administration is safe in patients with ARDS, with little or no infusion reaction and few late adverse effects. However, due to the relatively small number of patients that have received MSC therapy, further investigations are still required to detect efficacy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Cruz FF, Weiss DJ, Rocco PR. Prospects and progress in cell therapy for acute respiratory distress syndrome. Expert Opin Biol Ther 2016;16:1353-60. [Crossref] [PubMed]
- Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med 2014;2:1016-26. [Crossref] [PubMed]
- Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 2015;3:24-32. [Crossref] [PubMed]
- Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med 2019;7:154-62. [Crossref] [PubMed]
- Martínez-Peinado P, Pascual-García S, Roche E, et al. Differences of Clonogenic Mesenchymal Stem Cells on Immunomodulation of Lymphocyte Subsets. J Immunol Res 2018;2018:7232717. [Crossref] [PubMed]
- François M, Copland IB, Yuan S, et al. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 2012;14:147-52. [Crossref] [PubMed]
- Cruz FF, Borg ZD, Goodwin M, et al. Freshly thawed and continuously cultured human bone marrow-derived mesenchymal stromal cells comparably ameliorate allergic airways inflammation in immunocompetent mice. Stem Cells Transl Med 2015;4:615-24. [Crossref] [PubMed]
- Gramlich OW, Burand AJ, Brown AJ, et al. Cryopreserved Mesenchymal Stromal Cells Maintain Potency in a Retinal Ischemia/Reperfusion Injury Model: Toward an off-the-shelf Therapy. Sci Rep 2016;6:26463. [Crossref] [PubMed]
- Dang S, Yu ZM, Zhang CY, et al. Autophagy promotes apoptosis of mesenchymal stem cells under inflammatory microenvironment. Stem Cell Res Ther 2015;6:247. [Crossref] [PubMed]
- Zhu W, Chen J, Cong X, et al. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells 2006;24:416-25. [Crossref] [PubMed]
- Ghahremani Piraghaj M, Soudi S, Ghanbarian H, et al. Effect of efferocytosis of apoptotic mesenchymal stem cells (MSCs) on C57BL/6 peritoneal macrophages function. Life Sci 2018;212:203-12. [Crossref] [PubMed]
- de Witte SF, Luk F, Sierra Parraga JM, et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018;36:602-15. [Crossref] [PubMed]
- Silva LH, Antunes MA, Dos Santos CC, et al. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther 2018;9:45. [Crossref] [PubMed]
- Galleu A, Riffo-Vasquez Y, Trento C, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med 2017;9. [Crossref] [PubMed]
- Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 2014;15:39. [Crossref] [PubMed]
- Simonson OE, Mougiakakos D, Heldring N, et al. In Vivo Effects of Mesenchymal Stromal Cells in Two Patients With Severe Acute Respiratory Distress Syndrome. Stem Cells Transl Med 2016;5:845. [Crossref] [PubMed]


