
Combining current of injury and P-wave sensing optimized right atrial active-fixation leads implantation
Introduction
The Comprehensive Test of Phonological Processing (CTOPP) extended study revealed that there was a significant reduction in the incidence of atrial fibrillation with the application of physiological pacing (1). A secondary analysis from the mode selection trial (MOST) hypothesized that pacemaker patients with preserved left ventricular ejection fraction (LVEF) may also develop heart failure (HF), depending on the prevalence of right ventricular pacing (2). Accordingly, a number of different pacing algorithms have been developed to reduce the degree of ventricular pacing in the atrioventricular sequential pacing mode (DDD). These systems have been shown in clinical studies to reduce the risk in developing atrial fibrillation mainly in patients with sinus node disease (3-5). Atrial and ventricular lead stability is a prerequisite for ensuring physical pacing.
Lead implantation is inherently injurious to the focal myocardium, and it changes the electro-activity of the myocardium in a way similar to ischemic injury. This induced electro-activity is called current of injury (COI) (6,7). However, it is difficult to locate the ministry of the right ventricular outflow tract septal electrode. Furthermore, studies have revealed that the right ventricular outflow tract septal electrode can be well located in only 61% of these patients (8). In Saxonhouse et al.’s study, COI resolution was observed within a 10 min-recording time (9). Besides, Shali et al.’s work revealed that fully rotated leads were associated with the slowest COI recovery, and it also demonstrated that the time course of COI is correlated to acute lead stability in rabbits (10). In addition, recent studies on active-fixation leads have found that the magnitude of COI can predict acute active-fixation lead stability and threshold adequacy (9,11).
Poor P-wave or R-wave sensing would induce competitive cardiac pacing, causing rapid ventricular arrhythmia or rapid atrial arrhythmia, and even death. Low-pacing thresholds extend pacemaker life. In short, good P-wave or R-wave sensing and low-pacing thresholds allow the pacemaker to be used for a longer period. The midterm performance of active-fixation leads can be predicted through the COI recorded at the time of lead implantation, as reported by Haghjoo et al. (12).
Good chronic pacing parameters avoid lead replacement and reduce the power consumption of the pacemaker. These leads have a certain life, and good pacing parameters can extend the life of these leads. Compared with atrial lead implantation, ventricular lead implantation technology has become more mature. Due to anatomical differences between the ventricular and atrial myocardium, the fluctuation range of P-wave sensing is smaller than R-wave sensing. Thus, atrial leads are more susceptible to poor perception. Therefore, the present study aimed to investigate the conditions for optimizing right atrial active-fixation lead implantations, in order to obtain optimal chronic pacing parameters.
Methods
Selection criteria
Between July 2014 and October 2016, 98 consecutive patients, referred to our center for the implantation of dual-chamber pacemakers for symptomatic bradycardia (sick sinus syndrome, atrioventricular block, or both), who were undergoing active-fixation atrial pacing placed at the right atrial appendage, were selected for the present study.
Patients were excluded based on the following criteria: (I) an age <18 years old and a New York Heart Association (NYHA) heart function grade of III or IV; (II) presence of complex congenital heart disease; (III) inability to attend the outpatient device clinic for routine follow-up; (IV) severe liver or kidney damage; (V) presence of atrial fibrillation during the implantation process, in which the pacing threshold could not be measured; (VI) presence of atrial fibrillation during the 3-month pacemaker device follow-up, in which the pacing threshold could not be measured.
The study protocol was approved by the Ethics Committee of Fujian Medical University Union Hospital (No. 2017KY011), and a written informed consent was obtained from all patients.
Implantation technique
Implantation of the right atrial active-fixation pacing leads
The devices were implanted in the Electrophysiology Laboratory using standard implant techniques with local anesthetic and conscious sedation. The leads were inserted through the left or right subclavian vein. The right atrial active-fixation pacing lead was fixed after stable implantation of the ventricular pacing lead. The right atrial active-fixation pacing lead was placed in the right atrial appendage. The active fixed pacing lead model was the St. Judea 1888Tc or Medtronic 5076-52. Once the proper location was fluoroscopically identified, the helix was extended according to the recommendations of the related companies. The intracardiac electrogram was recorded at 25 mm/s from the Bard multi-channel electrophysiology (EP) recording system after the end of the fixed right atrial lead was linked to the V1 lead of EP. The PR-segment elevation (COI) was measured at the 0- and 10-minute point of the atrial lead fixation (Figure 1). In order to allow for the decrease in pacing threshold and impedance, pacing parameters were measured at 0 minute and after 10 minutes of atrial lead fixation. The atrial leads were all of the bipolar, steroid-eluting and extendable-retractable type, with an electrically active helix.
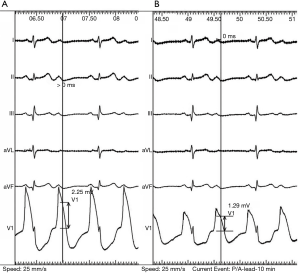
Follow-up and data collection
All patients were followed up for at least 3 months. Atrial pacing parameters included P-wave sensing, pacing threshold and lead impedance, and were measured after 3 months. After 3 months of follow-up, the atrial leads were considered as the “optimized group” when these leads had a P-wave sensing of ≥2.0 mV, a pacing threshold of ≤1.0 V, a lead impedance within 300–1,000 ohms, and no dislodgment. Otherwise, the atrial leads were considered as the “conventional group”.
The data of all patients including age, gender, preoperative diagnosis and preoperative echocardiography were collected.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software. Normal distribution data were expressed as mean ± standard deviation (SD), and the two-sample t-test was used to compare the data. Otherwise, non-normal distribution data were expressed as median [quartile range (QR)], and were compared using the nonparametric Mann-Whitney U test. Chi-square test was used to compare the count data. A binary logistic regression analysis model was established to identify the predictors of the optimized group. Hosmer-Lemeshow statistics was used to confirm the model fitness for the data. The sensitivity and specificity of each variable was determined using the receiver-operating characteristic (ROC) curve and the standard formula. Statistical significance was assumed at P<0.05.
Results
Baseline characteristics
All 98 patients implanted with active-fixation atrial leads completed the 3-month follow-up, in which 67 patients were assigned to the optimized group and 31 patients were assigned to the conventional group after 3 months of follow-up. Among these 98 patients, 55 (56%) patients were male and 43 (44%) patients were female. The mean age of these patients at implantation was 63±12 years old. Indications for the pacemaker were atrioventricular block in 32 patients and sick sinus syndrome in 66 patients (Table 1).
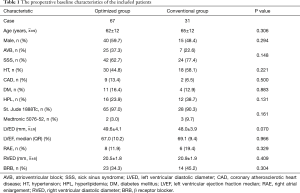
Full table
Atrial leads
Leads in the optimized group had a higher COI at 0 minute [COI0min, 2.06 (1.10) vs. 1.29 (1.42) mV, P=0.009] and COI at 10 minutes [COI10min, 1.23 (1.38) vs. 0.71 (0.61) mV, P=0.005] in the electrical measurements of the implantation time. P-wave sensing measured at 0 minute [P0min, 4.0 (2.2) vs. 2.4 (2.7) mV, P=0.005] and after 10 minutes [P10min, 4.0 (2.7) vs. 2.4 (1.7) mV, P<0.001] after lead fixation were also significantly higher in leads in the optimized group than in the conventional group. Pacing threshold measured at 0 minute [T0min, 0.8 (0.6) vs. 1.0 (0.4) V, P=0.011] and after 10 minutes [T10min, 0.7 (0.4) vs. 0.8 (0.5) V, P=0.014] of lead fixation were significantly lower in leads in the optimized group, compared to the conventional group (Table 2).

Full table
However, pacing impedance at 0 minute {IMP0min, 640 [160] vs. 640 [140] ohms, P=0.833} and after 10 minutes {IMP10min, 640 [120] vs. 600 [120] ohms, P=0.221} were similar in leads between the optimized group and conventional group (Table 2).
Predictors of outcome for atrial leads in the optimized group
Among multiple implant pacing parameters, active-fixation atrial leads after 3 months in the optimized group was correlated with COI10min [odds ratio (OR): 0.296, 95% confidence interval (CI): 0.093–0.939, P=0.039] and P10min [OR: 0.449, 95% CI: 0.265–0.762, P=0.003] (Table 3).
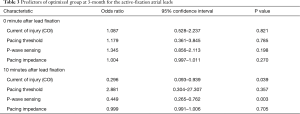
Full table
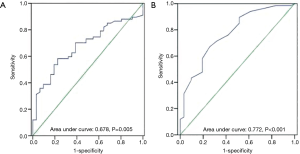
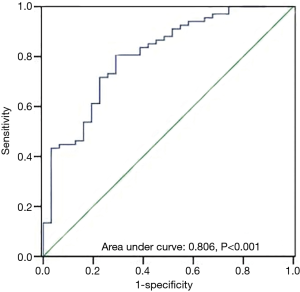
The ROC curve analysis was performed on atrial COI10min and P10min to define the optimal cut-off values for the prediction of the optimized leads. The ROC curve analysis revealed that COI10min ≥1.04 mV predicted for the optimized lead after 3 months with a sensitivity of 0.58 and a specificity of 0.77 (Figure 2A). In addition, P10min ≥3.3 mV was identified as the optimal cut-off (sensitivity: 0.67; specificity: 0.74) to predict the optimized lead at 3 months (Figure 2B). Moreover, with the combined COI10min ≥1.04 mV and P10min ≥3.3 mV as the predictable criteria, the area under the ROC curve was 0.806 (sensitivity: 0.70; specificity: 0.77) (Figure 3).
Discussion
The main finding of the present study was that COI and P-wave sensing recorded after 10 minutes of the lead fixation may predict the optimized lead for active-fixation atrial leads after 3 months. Kashiwase et al.’s study suggested that the threshold descends and approaches a 5-minute stable plateau after implantation with active-fixation (13). The ROC curve analysis revealed that COI10min of ≥1.04 mV indicated the optimized lead after 3 months with a sensitivity of 0.58 and a specificity of 0.77, and the area under the ROC curve was 0.678. The predictive optimized lead after 3 months with COI10min had low sensitivity, and was not recommended to be used alone. In addition, the optimized lead after 3 months could be predicted by P10min of ≥3.3 mV (sensitivity: 0.67; specificity: 0.74), and the area under the ROC curve was 0.772. The sensitivity of P10min was higher, when compared to the sensitivity of COI10min, and the specificity of these two was similar.
However, with the combined COI10min ≥1.04 mV and P10min ≥3.3 mV as the predictable criteria, the area under the ROC curve was 0.806 (sensitivity: 0.70; specificity: 0.77). Compared with the respective prediction, the sensitivity and specificity of the combined prediction were enhanced.
In Mond et al.’s study, the pacemaker threshold peak appeared at 1 month after the operation (14). The study conducted by Haghjoo et al. (12) demonstrated that lead performance at 6 months can be predicted through an adequate amount of COI recorded at the time of lead implantation. The adequate amount of COI was defined as an increase in PR-segment elevation of ≥2.0 mV for atrial leads. The conclusion of the above study was different from that in the present study.
The main differences between the study of Haghjoo et al. (12) and the present study were as follows: the study of Haghjoo et al. (12) defined “good performer at 6 months of follow-up” as that having a P-wave sensing of ≥1.5 mV, a pacing threshold of <1.5 V, and no dislodgment, while in the present study patients were followed up for only 3 months. The study of Kistler et al. (15) demonstrated that the active-fixation leads maintained stable long-term pacing parameters after 3 months following implantation. The data from the follow-ups were kept stable during the 12 months post-implant (16). J-shaped and straight atrial leads with active (screw-in) fixation mechanism demonstrated favorable lead performance throughout follow-up (17). Therefore, pacing parameters at 3 and 6 months following implantation can both represent the chronic pacing performance. The present study defined “optimized performer after 3 months of follow-up” as those having a P-wave sensing of ≥2.0 mV, a pacing threshold of ≤1.0 V, a lead impedance within 300–1,000 ohms, and no dislodgment. The present study requires a higher standard of pacing threshold and a P-wave sensing level, as both are important for prolonging the service life of pacemakers and pacing electrodes. High P-wave sensing can reduce the incidence of competitive cardiac pacing, and ensure that the pacemaker works better (2). The present study did not record COI10min. The study conducted by Chen et al. (18) demonstrated that a low level of COI0min and COI10min suggest a poor lead fixation, which shows the importance of COI within 10 minutes after lead fixation. The study conducted by Redfearn et al. (11) revealed that the continuous monitoring of lead parameters within 10 minutes of fixation is useful for predicting acute lead stability, and the adequacy COI can predict for acute lead stability and acute pacing thresholds. Hence, it is necessary to dynamically monitor these pacing parameters during lead implantation.
In the study conducted by Chen et al. (19), the optimized placement of a right ventricular lead was identified by COI0min >4.77 mV and R-wave sensing >7.25 mV recorded after 10 minutes of lead fixation. The optimized ventricular lead was defined as an R-wave sensing of >5.0 mV, a pacing threshold of <1.4 V, and a lead impedance of within 300–1,500 ohms after 10 minutes of lead fixation. Similarly, it was found that high chronic P-wave sensing was correlated to high P10min.
The results of the present study should be interpreted in light of certain limitations. First, the sample size of the study was relatively small. Second, the present study had a retrospective design, which selects the case of past patients in our center; hence, there is a certain choice bias.
Conclusion and clinical implications
The present study suggests that right atrial lead implantation parameters might be associated with lead optimized performance after 3 months of follow-up. The chronic performance of right atrial active-fixation leads may be predictable using the COI and P-wave sensing recorded at 10 minutes after lead fixation. COI ≥1.04 mV and P-wave sensing ≥3.3 mV recorded at 10 minutes after atrial lead fixation are recommended to possibly optimize lead performance after 3 months.
For young patients who need to be implanted with permanent pacemakers, they may have to replace the pacemaker several times due to energy depletion. In addition, lead stability and good pacing parameters can extend the life of the lead and pacemaker. Therefore, the pacing lead implantation is particularly important.
In order to obtain long-term good pacing parameters and lead stability after lead implantation, the results of the present study need to be considered.
Acknowledgements
Funding: This study was sponsored and funded by Fujian Province Medical Innovation Project (2016-CX-17).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Ethics Committee of Fujian Medical University Union Hospital (No. 2017KY011), and a written informed consent was obtained from all patients.
References
- Kerr CR, Connolly SJ, Abdollah H, et al. Canadian Trial of Physiological Pacing: Effects of physiological pacing during long-term follow-up. Circulation 2004;109:357-62. [Crossref] [PubMed]
- Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932-7. [Crossref] [PubMed]
- Gross JN, Moser S, Benedek ZM, Andrews C, Furman S. DDD pacing mode survival in patients with a dual-chamber pacemaker. J Am Coll Cardiol 1992;19:1536-41. [Crossref] [PubMed]
- Veasey RA, Arya A, Silberbauer J, et al. The relationship between right ventricular pacing and atrial fibrillation burden and disease progression in patients with paroxysmal atrial fibrillation: the long-MinVPACE study. Europace 2011;13:815-20. [Crossref] [PubMed]
- Sweeney MO, Bank AJ, Nsah E, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med 2007;357:1000-8. [Crossref] [PubMed]
- Grossi EA, Parish MA, Kralik MR, et al. Direct-current injury from external pacemaker results in tissue electrolysis. Ann Thorac Surg 1993;56:156-7. [Crossref] [PubMed]
- Varriale P, Niznik J. Unipolar ventricular electrogram in the diagnosis of right ventricular ischemic injury. Pacing Clin Electrophysiol 1978;1:335-41. [Crossref] [PubMed]
- Rosso R, Medi C, Teh AW, et al. Right ventricular septal pacing: a comparative study of outflow tract and mid ventricular sites. Pacing Clin Electrophysiol 2010;33:1169-73. [Crossref] [PubMed]
- Saxonhouse SJ, Conti JB, Curtis AB. Current of injury predicts adequate active lead fixation in permanent pacemaker/defibrillation leads. J Am Coll Cardiol 2005;45:412-7. [Crossref] [PubMed]
- Shali S, Wushou A, Liu E, et al. Time course of current of injury is related to acute stability of active-fixation pacing leads in rabbits. PLoS One 2013;8:e57727. [Crossref] [PubMed]
- Redfearn DP, Gula LJ, Krahn AD, et al. Current of injury predicts acute performance of catheter-delivered active fixation pacing leads. Pacing Clin Electrophysiol 2007;30:1438-44. [Crossref] [PubMed]
- Haghjoo M, Mollazadeh R, Aslani A, et al. Prediction of midterm performance of active-fixation leads using current of injury. Pacing Clin Electrophysiol 2014;37:231-6. [Crossref] [PubMed]
- Kashiwase K, Kobayashi H, Hirata A, et al. Acute changes in the pacing threshold after lead implantation. Comparison between retractable and sweet-tip active-fixation leads. Int Heart J 2012;53:108-12. [Crossref] [PubMed]
- Mond HG, Helland JR, Stokes K, et al. The electrode-tissue interface: the revolutionary role of steroid-elution. Pacing Clin Electrophysiol 2014;37:1232-49. [Crossref] [PubMed]
- Kistler PM, Liew G, Mond HG. Long-term performance of active-fixation pacing leads: a prospective study. Pacing Clin Electrophysiol 2006;29:226-30. [Crossref] [PubMed]
- Scherer M, Ezziddin K, Klesius A, et al. Extension of generator longevity by use of high impedance ventricular leads. Pacing Clin Electrophysiol 2001;24:206-11. [Crossref] [PubMed]
- Luria D, Bar-Lev D, Gurevitz O, et al. Long-term performance of screw-in atrial pacing leads: a randomized comparison of J-shaped and straight leads. Pacing Clin Electrophysiol 2005;28:898-902. [Crossref] [PubMed]
- Chen JH, Zhang FL, Chen XH, et al. Dynamic changes in current of injury predicts adequate active lead fixation in permanent pacemaker leads. Journal of Clinical Cardiology 2013;29:819-23.
- Chen JH, Zhang FL, Fan L, et al. Ideal current of injury and R-wave sensingvalues for identifying optimized placement of right ventricular active-fixation pacing leads. Zhonghua Xin Xue Guan Bing Za Zhi 2016;44:406-10. [PubMed]


