Influence of prophylactic antibiotic duration on postoperative pneumonia following pulmonary lobectomy for non-small cell lung cancer
Introduction
Although opportunities for radical surgery for non-small cell lung cancer (NSCLC) have increased in recent years, improvements in perioperative management, in addition to minimally invasive surgery, is important in order to improve the surgical outcome. Postoperative infectious complications include surgical site infection (SSI) and postoperative pneumonia, which is classified as a remote organ infection. Postoperative pneumonia after pulmonary resection occasionally becomes severe and may result in mortality in 12.6% of cases (1); in fact, it is the most frequent infectious disease cause of death after pulmonary resection. Therefore, overcoming postoperative pneumonia after pulmonary resection is extremely important to improve surgical outcome.
The usefulness of prophylactic antibiotic administration in the field of pulmonary surgery has been reported in the 1970s (2). According to that prospective comparison study between cefazolin and placebo, prophylactic antibiotics reduced the incidence of wound infection and reduced the incidence of postoperative empyema. Although reduction in the incidence of wound infection was reconfirmed by the subsequent clinical trials, the factors contributing to the reduction in the incidence of postoperative pneumonia were not clear (3-5). Therefore, the effect of perioperative prophylactic antibiotic administration according to the current guidelines is limited to SSIs, and the period of administration of prophylactic antibiotics may be different between SSI and postoperative pneumonia.
The duration of postoperative prophylactic antibiotics administration recommended by several guidelines, including the Center for Disease Control and Prevention (CDC) guideline, were based on evidence from SSI cases (6). From the viewpoint of the drug resistance, minimizing postoperative prophylactic antibiotic administration is desired. However, the appropriate period of prophylactic antibiotic administration for postoperative pneumonia has yet to be elucidated. This study aimed to evaluate the influence of the duration of prophylactic antibiotics on postoperative pneumonia following pulmonary lobectomy for NSCLC in patients who received antibiotics intraoperatively (short period) and in those who received antibiotics both intraoperatively and postoperatively (long period).
Methods
Patient selection
This retrospective cohort study reviewed the medical records of all patients who underwent radical lobectomy for NSCLC between January 2014 and June 2018 at Iwate Medical University Department of Thoracic Surgery. This study was approved by the Iwate Medical University institutional review board. Comprehensive informed consent was obtained for this retrospective medical record review (permit number: MH2018-565).
At our institute, the surgical indications for complete video-assisted thoracoscopic surgery (VATS) comprise the concept of a thoracoscopically resectable lesion, covering almost 90% of surgical patients with NSCLC. Patients received preoperative chemotherapy or radiation, cases of pneumonectomy, bilobectomy, sublobar resection, and concomitant resection with the thoracic chest wall were excluded. All patients were given complete preoperative pulmonary evaluation, including blood tests (i.e., albumin, etc.) 2 days before surgery. The patients with chronic obstructive pulmonary disease (COPD) were instructed to continue their inhaled medications preoperatively. In all patients, smoking was stopped for at least 8 weeks before surgery. Routine sputum culture monitoring has not performed before surgery and intraoperatively.
Prolonged air leak was defined as air leakage lasting 7 days or more (7). The definition of postoperative pneumonia in this study was based on a previous report (8). Briefly, it was defined as Clavien-Dindo classification grade II or higher and the presence of (I) abnormal radiographic findings (i.e., new or changing radiographic infiltrates that persisted after physiotherapy); (II) fever greater than 38 °C; and (III) one of the following criteria: a new rise in C-reactive protein or white blood cell count (WBC, >10,000/dL) over the last 24 hours or an increase and change in the sputum, possibly with purulence. If atelectasis and one of the above conditions were combined, it was classified as pneumonia. Patients with acute exacerbation of interstitial pneumonia were excluded. Finally, 477 patients met the selection criteria.
Pulmonary function testing
At our institute, pulmonary function was tested using a spirometer (CHESTAC 8800, CHEST M. I. Inc., Tokyo, Japan), according to American Thoracic Society standards (9). The vital capacity (VC), forced expiratory volume in 1 second (FEV1), and diffusing capacity for carbon monoxide (DLCO) were measured preoperatively within 1 month before surgery. The percent predicted DLCO (%DLCO) was calculated by Burrows formula (10). Predicted postoperative pulmonary function was calculated according to the formula described in a previous report (11), based on the number of segments that remained after surgery. The number of pulmonary segments considered was 5 for right lower lobe, 3 for the right upper lobe, 2 for the right middle lobe, and 4 for the left upper and lower lobe. Each segment was considered to represent 1/19 of the preoperative function.
Preoperative management
Preoperative inspiratory exercise was performed in all patients with the use of incentive spirometer (Tri-ball Z, Medtronic, Minneapolis, MN, USA) which consists of three chambers and small balls within the chambers that can be raised by inhalation, that started at least 2 weeks before surgery, and continued after surgery until discharge. The training was performed almost 4 times a day with a set of 20 breaths, briefly the patients were instructed to take slow maximal inspirations and to hold each breath for as long as possible. During the preoperative hospitalization, the patients were trained to master adequate breathing and coughing techniques, instructed on incentive respiratory exercise again, and practiced peripheral muscle exercise training under physiotherapist supervision.
Surgical procedures
Pulmonary lobectomy was performed under general anesthesia with a double-lumen endotracheal tube for single lung ventilation. The affected lung was deflated as soon as the pleural space was opened, and deflation was maintained throughout most of the operative period. The fraction of inspired oxygen (FiO2) during surgery ranged from 0.3 to 1.0, based on intraoperative blood gas analysis evaluation. The open method was performed via posterolateral thoracotomy (6–12-cm long), dividing portions of the latissimus dorsi and anterior serratus muscles. Complete VATS lobectomy was performed via three ports with monitor vision only. Complete and systematic hilar and mediastinal lymph node dissection was performed in all cases. After completing the procedure, sealing test was performed before wound closure and was confirmed during reinflation of the affected lung. A chest tube (Blake®, 19 Fr, Ethicon, Somerville, NJ, USA) was placed from the 5th intercostal trocar placed to the apex.
Administration of prophylactic antibiotics
At our institute, prophylactic antibiotics were administered intraoperatively and postoperatively up to March 2017. Briefly, all patients received cefazolin at a dosage of 1 g intravenously after induction of general anesthesia, prior to skin incision. After surgery, prophylactic antibiotics were administered intravenously at a dosage of 1 g on the surgical day at ICU, and then twice a day until 72 hours after surgery (long period group, n=309). From April 2017, based on our institute’s protocol on the proper use of antibiotics, prophylactic antibiotic administration was limited to intraoperative dose only, that is, prior to skin incision and at 3 hours after starting surgery (short period group, n=168). In this study period, there were no patients suspected to have allergy against cefazolin.
Postoperative management
C-reactive protein and WBC were measured on postoperative days 0, 1, 2, 4, and 6 or 7. A chest X-ray was obtained daily. The chest tube was suctioned at −5 cmH2O on the morning of postoperative day 1. The criteria for chest tube removal were: absence of air leakage through the chest tube at the time of evaluation; pleural fluid drainage less than 200 mL/24 hours; and the absence of pneumothorax on postoperative chest X-ray. On the morning after chest tube removal, a chest X-ray was performed to rule out the occurrence of pneumothorax. Postoperative pulmonary rehabilitation was started as early as postoperative day 1 in all patients. Briefly, diaphragmatic breathing exercises, peripheral circulation exercises, and exercises for chest expansion and shoulder girdle mobilization were introduced. Inspiratory exercise with the use of incentive spirometer was also continued. The day after removing chest drain, peripheral muscle exercise training including a cycle ergometer was performed under physiotherapist supervision. The patients themselves continued the training involving breathing and coughing techniques until discharge.
Routine postoperative pain management was performed in all patients of both groups. Briefly, oral analgesia was started 6 hours after surgery and typically included 60 mg loxoprofen 3 times per day, sometimes with 25 mg diclofenac suppository 1 to 2 times per day, as needed. The patients were discharged if there were no complications during the postoperative period. Our institutional standard protocol was to follow up all patients every 3 to 6 months after surgery for 5 years.
Statistical analysis
JMP 12.2.0 (SAS Institute, Cary, NC, USA) statistical software was used for statistical analysis. Groups were compared using the Pearson chi-square test or Wilcoxon’s rank sum test. To control for potential differences in the preoperative characteristics of the patients in the two groups, a propensity score matching method was used. The propensity scores were generated using logistic regression, based on clinically relevant preoperative variables, such as age, gender, body mass index (BMI), pack years smoked (Brinkman index), presence of COPD (FEV1 <70%), presence of usual interstitial pneumonia (UIP) on preoperative chest computed tomography (CT), history of steroid therapy, cerebrovascular disease, diabetes mellitus, tumor size, and surgical approach. There variables were considered possible confounders because of their potential association with postoperative pneumonia, based on clinical knowledge. Patients were matched 1:1 by nearest neighbor matching (caliper width: 0.2) without replacement. Comparisons between the matched groups were performed with McNemar’s test for categorical variables and paired t-test or Wilcoxon’s sign rank test for continuous variables, appropriately. The standardized difference was used to measure covariate balance, whereby an absolute standardized difference above 0.1 represented a meaningful imbalance.
The multivariate predictors were evaluated using logistic regression analysis, and the odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. On logistic regression analysis, the conventional receiver operating characteristic (ROC) curve was used to determine the cut-off value of each variable that gave the maximal sensitivity and specificity on predicting postoperative pneumonia. Differences between groups were considered significant at P<0.05. The continuous data were expressed as mean ± standard deviation. Categorical data were expressed as counts and proportions.
Results
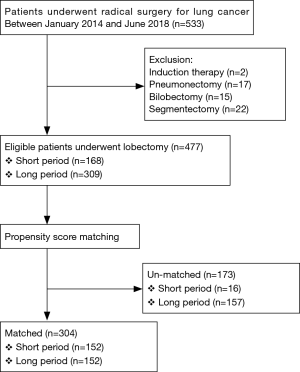
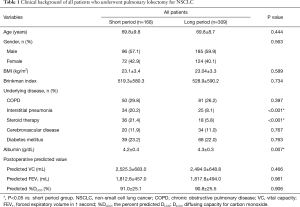
On retrospective review, 533 patients underwent radical surgery for NSCLC during the study period. After exclusion (Figure 1), a total of 477 patients (168 patients in the short period group and 309 patients in the long period group) were enrolled in this study. The clinical characteristics of the study population are summarized in Table 1. There was no significant difference between both groups in terms of age, gender, BMI, Brinkman index, and the incidence of COPD and diabetes mellitus. Compared with the short period group, the long period group had significantly low population of patients with steroid treatment history (5.8% vs. 21.4%, P<0.001) and coexistence of cerebrovascular disease (8.1% vs. 20.2%, P<0.001).
Full table
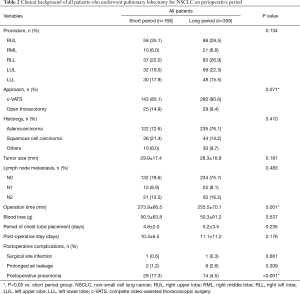
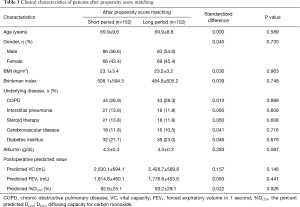
Table 2 shows the patients’ background during the perioperative period. There was no significant difference between both groups in terms of SSI rate (P=0.661). Compared with the short period group, the long period group had significantly shorter operation time (255.5±70.1 vs. 273.9±66.5 minutes, P=0.001) and significantly lower rate of postoperative pneumonia (4.5% vs. 17.3%, P<0.001). Propensity score analysis generated two well-matched pairs of 152 patients (Tables 3,4); 90.5% (152/168) in the short period group and 49.2% (152/309) in the long period group were matched. After matching, there were no significant differences between both groups in terms of all preoperative variables, such as age, gender, BMI, Brinkman index, and history of underlying disease, as well as in the perioperative variables, such as tumor size, lymph node metastasis, operation time, postoperative stay, serum albumin level, and predicted postoperative pulmonary function. The balance of each sample size was assessed by standardized differences, and its values for the preoperative and perioperative variables were almost under 0.1. The long period group remained to have a significantly lower rate of postoperative pneumonia, compared with that of the short period group (3.9% vs. 16.4%, P<0.001).
Full table
Full table

Full table
The results of the multivariate analysis of the independent predictors of postoperative pneumonia are shown in Table 5. Short period administration of prophylactic antibiotics was an independent factor related to postoperative pneumonia in the matched patients (OR 6.82, P<0.001).
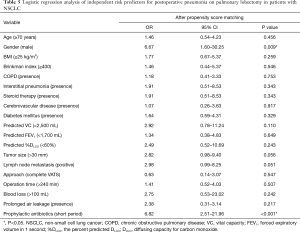
Full table
Discussion
This study evaluated the influence of the duration of prophylactic antibiotic administration in patients who underwent surgery for NSCLC. Propensity score analysis generated two matched pairs of 152 patients in both the short and long period groups, and the incidence of postoperative pneumonia was significantly less in the long period group. Logistic regression analysis demonstrated that short period of prophylactic antibiotic administration was an independent risk factor for pneumonia after pulmonary lobectomy. This result indicated that long period of prophylactic antibiotic administration reduced the rate of postoperative pneumonia after pulmonary lobectomy for NSCLC.
Evidence on prophylactic antibiotic administration immediately before and during surgery had been based on wound infection experiments (12). According to this report, the effects of antibiotics last for only 3 to 4 hours after wound exposure to bacteria. Moreover, administrating antibiotics 48 hours after wound closure seemed to be of no use. Therefore, the CDC guideline recommended a method that can maintain effective blood and tissue concentration of antibiotics during surgery. Since then, perioperative prophylactic antibiotic administration for the prevention of SSI had been undertaken and standardized (6). In particular, pulmonary lobectomy for lung cancer with bronchial opening was classified as semi-clean surgery, for which first generation cephalosporin or sulbactam/ampicillin is the first choice for infection prevention (13-15). The first dose should be administered 30 minutes before the skin incision, in order to achieve an effective blood concentration at the time of surgery; additional administration should be made after 3 hours to maintain the effective intraoperative blood concentration of antibiotics, and it can be administered only on the day of surgery if there is no symptom of infection (16,17). Postoperative pneumonia is classified as a remote organ infection, rather than an SSI. In a previous report on the use of broad-spectrum penicillin as a prophylactic antibiotic in patients who underwent pulmonary lobectomy, postoperative pneumonia developed in 8.7% (18). This rate was almost consistent with our 9.0% incidence of postoperative pneumonia in all eligible patients. Similar to the other studies, this study showed that postoperative pneumonia was more frequent than SSI, among the infectious diseases after pulmonary lobectomy in patents with NSCLC. In both groups in this study, the occurrence of SSI was under 1%, implying that a short period of prophylactic antibiotic administration was sufficient for SSI. On the other hand, a long period of prophylactic antibiotic administration was effective for the prevention of postoperative pneumonia. Adherence to the current practice guidelines should account for (I) the recommendations for pulmonary surgery, (II) the existence of a verified clinical trial, and (III) whether the cases focused on SSI alone or with other complications. Nevertheless, extending the use of prophylactic antibiotics after surgery may cause the emergence of resistant bacteria; therefore, it should be used judiciously (19,20).
According to a prospective study, intravenous sulbactam plus ampicillin was significantly more effective than cefazolin as a single shot for antibiotic prophylaxis after pulmonary resection (15). In this study, cefazolin was used based on evidence from SSI cases, and long period of administration was shown to be effective for the prevention of postoperative pneumonia. Based on this study, we administered cefazolin on the day of surgery, followed by oral administration of penicillin 2 days after surgery. In the future, we plan to evaluate the usefulness of oral administration of penicillin after surgery for postoperative pneumonia on a large number of cases.
Postoperative pulmonary fistula, particularly a large fistulous empyema, can complicate pneumonia by aspiration of pleural effusion. However, this study demonstrated that prolonged air leakage was not a significant risk factor for postoperative pneumonia (OR 2.38, P=0.217). This implied that the cause of postoperative pneumonia was not from an external factor but from an internal factor. However, the details are unknown at the present time, so further investigation is needed.
In recent years, attention has been drawn on the importance of prevention of postoperative pulmonary complications in elderly people. In the elderly, decrease in VC, increase in residual volume, attenuation of the cough reflex, reduction of swallowing reflex, etc. were reported as risk factors for postoperative pneumonia (6,21). Preoperative pulmonary rehabilitation was also reported to reduce the incidence of postoperative pulmonary complications (22). Furthermore, the resident bacteria of the oral cavity had been noted as a cause of postoperative pneumonia, and the importance of preoperative oral care had been emphasized as one of the measures for postoperative pneumonia prevention (23). Comprehensive perioperative respiratory care that aims to reduce these postoperative pulmonary complications should be further examined in the future.
Our study had several limitations, including its retrospective nature and the small number of patients in a single institute. Also, it is difficult to evaluate postoperative management according to the background of the era. To obtain strong conclusions, a randomized study is essential. Propensity score matching is one of the effective methods when, to some extent, prediction is already attached to the results and a random comparison test is difficult to perform ethically. Notably, not all of the preoperative differences between the groups were compensated for by the propensity score matching, despite efforts to control the possible biases. Nevertheless, we believe that our findings could contribute to the improvement of the quality of pulmonary lobectomy in patients with NSCLC. In addition, we emphasize that the results of this study do not recommend inappropriate use of prophylactic antibiotics for a long period of time after surgery. A multicenter prospective study is required to validate our results in the future. In conclusion, prophylactic antibiotic administration in both the intraoperative and postoperative periods reduced the incidence of pneumonia after pulmonary lobectomy for NSCLC.
Acknowledgements
The authors thank Dr. Eri Maeda for her suggestion and support on statistical analysis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethics approvals for the protocol of this study was obtained from the Ethics Committee of Iwate Medical University. Comprehensive informed consent was obtained for this retrospective medical record review (permit number: MH2018-565). Before enrollment, the comprehensive written informed consents were obtained.
References
- Committee for Scientific Affairs. Thoracic and cardiovascular surgery in Japan during 2015: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2018;66:581-615. [Crossref] [PubMed]
- Kvale PA, Ranga V, Kopacz M, et al. Pulmonary resection. South Med J 1977;70:64-8. [Crossref] [PubMed]
- Ilves R, Cooper JD, Todd TR, et al. Prospective, randomized, double-blind study using prophylactic cephalothin for major, elective, general thoracic operations. J Thorac Cardiovasc Surg 1981;81:813-7. [PubMed]
- Aznar R, Mateu M, Miró JM, et al. Antibiotic prophylaxis in non-cardiac thoracic surgery: cefazolin versus placebo. Eur J Cardiothorac Surg 1991;5:515-8. [Crossref] [PubMed]
- Bernard A, Pillet M, Goudet P, et al. Antibiotic prophylaxis in pulmonary surgery. A prospective randomized double-blind trial of flash cefuroxime versus forty-eight-hour cefuroxime. J Thorac Cardiovasc Surg 1994;107:896-900. [PubMed]
- Berríos-Torres SI, Umscheid CA, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017;152:784-91. [Crossref] [PubMed]
- Rivera C, Bernard A, Falcoz PE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011;92:1062-8. [Crossref] [PubMed]
- Schussler O, Alifano M, Dermine H, et al. Postoperative pneumonia after major lung resection. Am J Respir Crit Care Med 2006;173:1161-9. [Crossref] [PubMed]
- Standardization of spirometry--1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis 1987;136:1285-98. [Crossref] [PubMed]
- Burrows B, Kasik JE, Niden AH, et al. Clinical usefulness of the single-breath pulmonary diffusing capacity test. Am Rev Respir Dis 1961;84:789-806. [PubMed]
- Zeiher BG, Gross TJ, Kern JA, et al. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest 1995;108:68-72. [Crossref] [PubMed]
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 1961;50:161-8. [PubMed]
- Nichols RL. Antibiotic prophylaxis in surgery. J Chemother 1989;1:170-8. [Crossref] [PubMed]
- Bumpous JM, Johnson JT. The infected wound and its management. Otolaryngol Clin North Am 1995;28:987-1001. [PubMed]
- Boldt J, Piper S, Uphus D, et al. Preoperative microbiologic screening and antibiotic prophylaxis in pulmonary resection operations. Ann Thorac Surg 1999;68:208-11. [Crossref] [PubMed]
- Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med 1992;326:281-6. [Crossref] [PubMed]
- Page CP, Bohnen JM, Fletcher JR, et al. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg 1993;128:79-88. [Crossref] [PubMed]
- Schussler O, Dermine H, Alifano M, et al. Should we change antibiotic prophylaxis for lung surgery? Postoperative pneumonia is the critical issue. Ann Thorac Surg 2008;86:1727-33. [Crossref] [PubMed]
- Harbarth S, Samore MH, Lichtenberg D, et al. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 2000;101:2916-21. [Crossref] [PubMed]
- Hecker MT, Aron DC, Patel NP, et al. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med 2003;163:972-8. [Crossref] [PubMed]
- Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1-36. [PubMed]
- Saito H, Hatakeyama K, Konno H, et al. Impact of pulmonary rehabilitation on postoperative complications in patients with lung cancer and chronic obstructive pulmonary disease. Thorac Cancer 2017;8:451-60. [Crossref] [PubMed]
- Ishimaru M, Matsui H, Ono S, et al. Preoperative oral care and effect on postoperative complications after major cancer surgery. Br J Surg 2018;105:1688-96. [Crossref] [PubMed]